The lead acid battery is the current source that powers the starter when the engine starts. The battery is the main source of energy. Among the advantages of this type of battery are an extended service life of more than 500 cycles, high power density, and cost. In addition to vehicles, lead batteries are found in standby and emergency power sources of stationary and mobile types.
The operating principle of a lead-acid battery is to convert chemical and electrical energy.
Device
Structurally, an acid battery consists of the following elements:
- Frame. It is a monoblock. Batteries are placed in it. Made from acid resistant insulating material. As a rule, this is propylene.
- Electrode (grid coated with lead oxide powder paste). The positive electrodes are coated with lead dioxide. Negatively charged lattices are covered with a mass of spongy lead. In order to increase the anti-corrosion resistance of electrodes, a number of manufacturers add passivators to the lead-calcium alloy
- Separator-envelope. Installed to insulate grids and plates of opposite polarity in battery cells. If plates of different polarities come into contact, a short circuit will occur. The main difficulty in the manufacture of separators: their material must protect against such a short circuit, while the current must pass unhindered through the electrolyte. A compromise solution and a decent material option is microporous plastic. Most lead-acid batteries use a lead-calcium alloy electrode as the active material. They have a minimum level of self-discharge.
- Tire
- Lid
- Outlet.
- Terminals. They provide direct connection between the battery and the starter, generator, and allow you to quickly install or dismantle the battery.
- Indicators of electrolyte density and battery charge.
Each modern battery consists of six acid batteries. A serial connection is used. Six 2-volt elements provide an output of 12 volts.
Types of Lead Acid Batteries
A battery can be qualified according to two criteria: functional purpose and installed electrolyte. According to their functional purpose, devices are of two types:
- Starter. Ensures the car engine starts and supplies current to the starter. Requires maintenance and ventilation.
- Traction. Devices for cyclic modes. They are used in situations where it is necessary to provide mechanisms with continuous energy (power supply in a constant mode). Most often they are installed on electric cars, tractors, and loaders.
As for the second classification feature - the type of electrolyte, it can be absorbable, liquid, or gel-like. In most cases we are dealing with a liquid medium. The electrodes are in an electrolyte made of sulfuric acid solution.
The lower the charge of the cell, the lower the density of the electrolyte. When fully charged, the density reaches 1.29 g/cm3. In a number of modern batteries, the electrolyte is retained in a special microporous material. In this case, we are talking about an absorbed electrolyte. The volume of electrolyte poured into the battery is added in the volume that the microporous material can absorb. Better acid absorption leads to increased efficiency of the active mass. This is very valuable if the battery powers a regenerative braking system or a stop-start system. But due to the high cost of batteries of this type, there is no mass transition to them yet. Most use traditional lead-acid batteries.
The most well known solutions are those that focus on absorbed electrolytes. These are batteries made using AGM technology. There is no maintenance required for this type of lead-acid battery. For installation, you do not need to ensure that the room is ventilated. The operation requires no maintenance. At the same time, in recharging mode (buffer mode), the batteries can last 12-18 years, in cyclic mode - less.
How to choose a battery
The choice of models presented in stores is large. To buy a lead-acid battery, it is recommended to pay attention to the following nuances:
- Size. When selecting a battery, the size is important, which should correspond to the niche in the engine compartment.
- Polarity. The location of the terminals of the new battery and the old one must match.
- Brand. Experts recommend choosing a battery for a car from a well-known manufacturer, which guarantees the quality of the product within the stated period.
- Type. Serviced or unserviced. In the second case, the battery does not require additional care, but if it is deeply overdischarged, it cannot be regenerated.
- Declared current. The higher the parameter, the lower the internal resistance, the better the model.
Due to its consumer characteristics, a lead-acid battery is practically the only option for installation in a car. At the same time, manufacturers offer hundreds of options, differing in price, technical characteristics, and aimed at different customers.
Battery operating principle
How does a car battery work?
- When charging, electrical energy is converted into chemical energy.
- During a discharge, chemical energy is converted into electrical energy.
- The battery operates cyclically.
Thus, the operating principle is based on the conversion of electrical energy into chemical energy (this process occurs during charging) and chemical energy into electrical energy (on the contrary, during discharge). And then this process repeats.
Operation is affected by ambient temperature. If the air temperature rises, the power supplied by the battery increases. It would seem that the efficiency problem has been solved. But not everything is so simple; at the same time, the battery’s tendency to self-discharge and corrosion increases significantly.
A decrease in temperature, in turn, leads to a decrease in discharge capacity, as well as a decrease in the density of the electrolyte.
Design
Acid batteries have not changed in their basic internal structure for more than a hundred years.
The battery design includes:
- The electrodes are in the form of flat grids of lead; lead dioxide powder (PbO2) is pressed into the cells at the anode, metal lead powder (Pb) is pressed into the cells at the cathode.
- The separator is a porous dielectric that separates the electrodes from each other, preventing short circuits.
- The electrolyte is sulfuric acid H2SO4 diluted with water (distilled), electrodes and a separator are placed in it. Maximum electrical conductivity is achieved at a temperature of 20°C and a sulfuric acid concentration of 35%, which means an electrolyte density of 1.26 g/cm³. In this case, the internal resistance is minimal, the losses inside the device are significantly small. In places with a low-temperature climate, it is possible to increase the density of the solution to 1.29 g/cm³ - 1.31 g/cm³. Increasing the concentration of the acid solution prevents the electrolyte from freezing and the formation of ice inside the case, which can damage the electrodes and rupture the battery.
Advantages and disadvantages
The popularity of lead-acid devices can easily be explained by many factors.
- Low cost and, accordingly, affordable price.
- Reliability.
- Low self-discharge.
- Easy to maintain (no need to add electrolyte), and for some models, such as solutions using AGM technology, no need for service at all.
However, humanity is constantly looking for an alternative, in some cases using alkali devices. Why? This is due to the fact that acid equipment also has disadvantages:
- Difficulties with organizing storage. Storage is only possible when charged.
- Poor overdischarge tolerance. Operating rules limit the number of charge-discharge cycles.
- Harmful to the environment (emission of fumes).
- High risks of overheating if charging incorrectly.
- Unstable operation at low temperatures. Sometimes this causes problems with starting the engine in winter.
- Weak ability to produce electric current for a long time in traction mode of operation.
GEL and AGM batteries
Many applications require batteries that are safe to use, completely sealed and acid resistant. They are called VRLA (valve regulated lead acid) batteries, sometimes also called SLA (sealed lead acid) batteries. The basis here is not only the use of sealed valves, but also trapping the electrolyte in the jars.
There are two known types of VRLA batteries - the more popular AGM and the much less common gel (GEL).
AGM stands for Absorbed Glass Mat. In the cells between the plates there is glass wool (mat), in which the electrolyte is retained. On the other hand, in gel batteries, the electrolyte is in the form of a gel due to the addition of silica.
The average user will not notice the difference between AGM and gel ones by eye, because their design and body may be the same. Often the AGM mark on the battery is missing; The inscription GEL is not always on the gel. These sealed batteries are mistakenly called "gel" batteries, although the most common type is AGM batteries.
The differences between AGM and gel batteries are mainly in durability and recommended charging and discharging conditions. Typically, most GELs cannot operate at high currents, so AGM batteries are typically used for UPSs, for example. On the other hand, GELs generally last longer, but only under milder operating conditions. True, there are gel batteries for heavy cyclic work and deep discharge.
It should be emphasized that the advantage of AGM and gel batteries is, on the one hand, their sealing and maintenance-free nature, and on the other hand, they require carefully selected charging conditions. Although they have valves, they will only work if they fail, when overcharging and severe gas formation occurs.
During proper charging, they release a certain amount of gases, the pressure increases, but due to the presence of a catalyst, recombination occurs, that is, the repeated conversion of hydrogen and oxygen into water. The valves are closed and open only with a particularly large increase in pressure, when strong gas formation occurs due to recharging.
Attention: even a single strong overcharge is very harmful and can irreversibly reduce the capacity of AGM and gel batteries. While outgassing was acceptable in the case of old classic open batteries, outgassing is prohibited in AGM and gel batteries.
Over-discharge is also very harmful, especially in AGM batteries. There are several adverse effects, including what is called sulfation, which is the formation of a non-conductive layer of lead sulfate crystals inside the battery. Even a single discharge to zero can cause severe, irreversible loss of capacity and such a discharged battery should be recharged as soon as possible.
Therefore, it is important not to overcharge or discharge the battery. It's actually simple. To prevent overcharging, it is enough to limit the charging voltage to a safe value. To prevent over-discharging, it is worth using a voltage drop alarm circuit and adding an automatic shutdown circuit.
Dependence of battery life on discharge depth
A car battery of a given capacity is definitely cheaper than a “non-car” battery of the same capacity. This is mainly due to the fact that a car battery is designed to operate in relatively mild conditions.
Although the temperature differences when using it are large, from -20 ° C to more than + 40 ° C, in the car it is constantly charged by the generator and regulator, then the voltage is kept at 14.4 V. Of course there is a high current for a few seconds during startup and small current when idle.
Accordingly, car batteries are of a relatively simple design and are not suitable for intensive cycling when they are almost completely discharged after charging. With intensive cycles (charge/full discharge) they will simply have a very short lifespan and will lose capacity after only a small number of cycles.
For heavy cyclic work, for example in electric forklifts or carts, so-called traction batteries are produced, which are more powerful, but also more expensive.
Reliability strongly depends on the depth of discharge. If we want to increase service life, we must get a battery with a larger capacity than required and never discharge it completely.
There are many types of "non-automatic" acid batteries. All are suitable for mild buffering conditions. But not all of them are suitable for heavy cyclic work - here you should use “reinforced” versions designed specifically for such work, which are more expensive. While, for example, a car battery with a capacity of 100 Ah can be bought for less than 10,000 rubles, for a deep-cycle (traction) battery of 12 V / 100 Ah you will have to pay from 20,000.
When it comes to the most popular sealed AGM batteries, many are designed primarily for use in backup circuits under relatively mild conditions. You can check in their documentation whether this type is also suitable for cyclic operation.
You also need to know that individual manufacturers offer several or even about a dozen series (families) of gel batteries, which differ, among other things, in their ability to operate cyclically and last for a long time.
Where are they used?
The solutions allow you to work with large peak power loads. Therefore, the scope of use of batteries is very wide. Installation possible:
- On all starter and a number of traction engines of cars.
- On motor vehicles: mopeds, motorcycles, ATVs, scooters.
- On boat motors.
- On aviation technology.
- Solutions with liquid electrolyte are more often used as starters, as buffers, and also on complex equipment, including aviation, they mainly use solutions absorbed on a separator (using AGM technology).
Where are they used?
Operational characteristics and design features allow lead-acid batteries to be widely used.
The main areas of use include:
- power supply for security and alarm systems;
- use as a source of starter current for a car;
- autonomous fire safety systems;
- emergency power systems in buildings and departments of medical institutions;
- cash registers and electric scales;
- uninterruptible power supply systems for computers;
- toys;
- light aircraft, etc.
Of course, motorists know lead-acid batteries as sources for supplying starter current. It’s not for nothing that batteries are called starter batteries.
But such batteries are not used as often as traction batteries, if we talk about powering cars.
Life time
Battery life is the potential time for which it will remain in working condition and within the parameters described for it by the manufacturer. This period actually means the maximum life of the battery under typical operating conditions. A number of factors can reduce the service life due to negligence. You need to be especially careful when starting up. It must be carried out in a stable environment. Vibration and temperature fluctuations are especially dangerous.
A sudden increase in temperature of 10°C will significantly reduce capacity by 25% and reduce service life by 50%. Reducing the temperature by the same amount will also reduce the battery capacity by about 25%. Compliance with the rules for storing lead-acid batteries is also very important. The room must be cool and dry. Stacking is prohibited.
Popular liquid electrolyte batteries usually last 3-4 years; when used in a stable environment and low loads, the possible service life is 7-8 years. Solutions made using AGM technology in recharging mode (buffer mode) can last 12-18 years.
The service life should never be confused with the warranty; the latter is always shorter. For example, for products that can last 4 years, the warranty period is usually 1-2 years.
Varieties
Based on the main characteristics relevant for lead-acid batteries, they can be divided into corresponding subcategories.
To begin with, it is worth focusing on the possibilities of their service. Here are the following types of lead-acid batteries:
- Serviced. Provides an open design. That is, the case is not sealed. The manufacturer installs special removable lids that allow access to the jars. Thus, you can add electrolyte or acid as necessary, visually check the liquid level and the condition of the plates, and take density measurements with a hydrometer.
- Maintenance free. The housing is completely sealed. Maintenance comes down only to monitoring the charge using an indicator, as well as connecting the battery to the charger.
The popularity of the latter is quite obvious. Maintained batteries are becoming less common as technology improves and motorists require less effort and attention to keep the battery in working condition.
Based on such basic characteristics as purpose and scope of application, the following types of lead-acid batteries are distinguished.
- Starter batteries . They can release a large amount of energy in a short time. Because of this, they have high self-discharge. Types of batteries required for cars. They need ventilation and some maintenance.
- Buffer . These types of lead-acid batteries are used to store a relatively small amount of energy for a short time. They operate in constant charging mode.
- For uninterrupted devices . Most often found in offices, they are intended for computer equipment. If a power outage occurs, they allow you to complete your work without losing data.
- For long-term energy supply . They are distinguished by their large weight and dimensions. But this allows consumers to be fed for a long time. Similar solutions can be found in the medical field, in intensive care units. They can power a fairly impressive number of consumers for a long time.
- Gel . There are 2 main technologies for the production of gel batteries. These are AGM and GEL. Characterized by a gel-like state of the electrolyte. Improved technology, although it is still based on lead and sulfuric acid. Gel batteries are used in cars, used to operate solar panels, etc.
To obtain certain characteristics, appropriate changes are made to the design.
When you need to deliver a lot of energy in a short time, the plates are made thin, but high and wide, reducing the distance between them. This allows them to release energy faster.
If little energy is consumed, but for a long time, then the plates should be thick, short and narrow, the distance between them increased.
The parameters of the battery are also influenced by the electrolyte and the alloying additives used, such as calcium, silver, zinc, etc. This allows us to distinguish several more varieties. Lead-calcium batteries are often found.
Charger
A car battery by itself cannot provide the car with the required amount of energy for a long time.
Therefore, it is important to take care of replenishing the energy used from the battery. This is where the charging system comes to the rescue. Its task is to produce electrical energy, which is supplied to all consumers of electrical energy of the vehicle during engine operation. How to physically charge a lead acid battery?
There are two options:
- Direct current.
- Alternating current.
The first option is easier to implement physically, but the second option is more efficient.
Therefore, at first, mainly a direct current generator was used for charging, and then it was replaced by an alternating current generator or alternator. It is considered the most promising charging option for modern vehicles. The need to switch from a DC generator to an alternator is due to the fact that there were difficulties with it in producing a large amount of energy at low engine speeds.
- The alternator rotor provides a rotating magnetic field inside the alternator.
The rotor field windings receive electric current through the brushes. The energy itself is produced in the stationary part, the stator. A special cooling fan draws in air and creates all the conditions to maintain normal temperature conditions for the diodes. In practice, when charging, the main thing is to read the manufacturer's instructions. The steps you need to take when charging may vary.
Battery recovery
Often there is a need to restore the battery.
If it sits down (this often happens after winter “downtime”), the main thing is not to panic. How to restore a lead acid battery practically? There are a number of recovery mechanics, but the simplest and most effective option is repeated low-current charging. The main trick: you need to take breaks between charges. Physics comes into play. The voltage increases, the perception of charge, on the contrary, decreases. During the pause, the electrode potentials reach the same level, the capacity of the lead-acid battery increases, and the density of the electrolyte also increases significantly. The result was achieved at a time when the density indicators can be considered normal for this type of battery.
Operating principle
The operating principle of a lead acid battery is as follows:
- The negative lattice reagent gradually disintegrates under the action of the electrolyte, forming lead ions. As a result of this decay, free electrons appear, which then enter the positive lattice of the electrode (via an external circuit);
- Lead ions react with the electrolyte to form lead sulfate. Due to its low solubility, it settles on the negative lattice.
- As a result, ordinary lead on the negative plate turns into sulfate.
- The positive electrode interacts less with the electrolyte than the negative electrode. Its main component, Pb02, reacts with water and is divided into positive and negative ions.
- The positive ones transmit the corresponding potential to the plate, where they merge with electrons. During the reduction reaction, Pb2+ is formed, which further reacts with the electrolyte.
- The resulting lead sulfate accumulates on the positive lattice, subsequently forming lead sulfate on its surface.
The battery receives electricity as follows:
- Near both plates the electrolyte contains a certain amount of water (H+, OH–) and lead sulfate (Pb2+, SO2/4)
- During the charging process, electrons move from an external power source from the positive terminal of the battery to the negative terminal.
- The incoming electrons restore the lead of the negative plate.
- The ions remaining after lead reduction and the H+ contained in the electrolyte combine to form H2SO
- On the positive plate, the incoming current knocks out 2 electrons from 2-valence lead, oxidizing it to 4-valence.
- As a result of subsequent interactions, Pb4+ combines with oxygen ions, reducing the material of the plus lattice.
- The remaining ions, reacting with each other, compensate for the density of the electrolyte.
Systematizing knowledge, acquiring skills: distance learning
So, you have become acquainted with the theory regarding batteries, classification, recovery methods, charging, but want to study the topic deeper, systematize your knowledge on this issue as much as possible, learn how to work with the battery in practice, test your knowledge?
Great! The ELECTUDE online training system for mechanics, electricians and diagnosticians will help you. The huge advantage of the online platform is that it is maximally focused on an integrated approach (basics of physics + practice-oriented materials), and you can get not just knowledge, but also specific skills, and the knowledge assessment system in the form of tests will help you really evaluate, what topics still need to be worked on, at what pace to move forward.
Below we provide screenshots and descriptions of the modules we translated into Russian, which directly relate to the topic discussed above.
Study, take a closer look at the ELECTUDE system and find a new source for yourself, not just for gaining knowledge, but for step-by-step development of practical skills. Indeed, in addition to the basic modules, the system has a special virtual simulator. It will help you “pump up” to the fullest. The system is excellent for distance learning and self-study.
Acid batteries; so that it is no longer disgusting to read what people write about them
I accidentally saw an article with comments on it, and anger began to boil inside me about the illiteracy of people in the field of acid (lead in common people) batteries that I could not stand it and decided to write to “geeks” (to be a geek, as it turns out, it’s not enough to buy an expensive phone) a short article about batteries. With consideration of those mistakes that constantly bother my eyes and cause a righteous desire to correct them. Let's start with the name. I very often see that the three letters A-K-B are used to describe everything that can be charged, absolutely any battery. People especially like to call Li-ion batteries with three letters. In fact, AKB is an abbreviation for Rechargeable Acid Battery. They mean only one type of battery - lead acid. From a modern point of view, this name causes some cognitive dissonance because at the moment the meaning of the word “battery” is galvanic cell that cannot be charged switched to the word “battery”. And it turns out that because of the word “rechargeable” it is a battery that can be charged, but because of the word “battery” it is as if it is a battery that cannot be charged. In reality, a battery is simply a chain of galvanic cells and only has a root in common with the word “battery”.
Next, let's move on to some myths, namely the main myth - the battery for a car has some significant differences from the battery for a UPS. And so you can’t use them both here and there. From a chemical point of view, any battery is absolutely the same
. How are they arranged? Very briefly - if the battery is charged, then one electrode is a lead grid with PbO2 paste applied to it, the second is the same grid with sponge lead paste. The electrolyte is a solution of sulfuric acid. During the discharge process, PbO2 is reduced and interacts with sulfuric acid to form PbSO4. Lead on the other electrode is oxidized and again forms PbSO4. At the end of the discharge we have both grid plates filled (more or less) with lead sulfate. When charging a battery, electrolysis occurs and lead sulfate again produces dioxide and metallic lead. Of course, here it is necessary to emphasize that the electrodes are not equal and their polarity should not be confused because Even at the production stage, appropriate additives are introduced into the electrode coating to improve their performance properties. Moreover, additives that are beneficial for one electrode are harmful for another. In very old times, somewhere at the beginning of the last century, in the conditions of simple batteries, it was probably permissible to reverse the polarity of the battery by mistake or for some purpose and it worked for some time after that. I doubt that it is acceptable now.
There are 6 such cells in a 12V battery, and 3 in a 6V battery. etc. Many people are confused by the battery voltage. Moreover, the values of the nominal voltage, charge, discharge. On the one hand, the batteries are called 12V (and 6V, 24V are also available, in my opinion, even 4V are occasionally found), but on the case of the same batteries for UPS, the manufacturer indicates a voltage higher than 13.5V.
For example:
Here we see that in forced mode the charge
maybe as much as 15V.
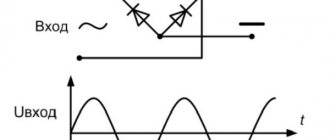
The voltage curve on the battery will explain everything:
On the left we see the voltage for a battery of 12 cells (24V nominal), 6 (12V nominal) and, most usefully, for a single cell. Areas of unwanted voltages during discharge/charge are also marked there. From the curve we can draw conclusions:
1 Voltage 12V, 24V, etc. are nominal and show only the number of galvanic cells (by dividing by two) in the battery. This is just a name for convenience.
2 The charging voltage can reach 2.5 V/cell, which for a 12V battery corresponds to 15V.
3 The voltage of a charged battery is considered acceptable at a value of 2.1-2.2 V/cell, which for a 12V battery corresponds to 12.6-13.2V.
Theoretically, the battery can be charged to 2.4 V/cell or even slightly higher, however, such charging will negatively affect both the condition of the electrodes and the electrolyte concentration. One day, before scrapping, I easily charged a 12V battery to a voltage of approx. 14.5V (I don’t remember the exact value).
So, the author of the article with which I started, decided that the charging voltage of a car battery and a battery from a UPS are different. This is not true, they have the same type of electrodes and the same concentration of sulfuric acid in the electrolyte (selected experimentally a long time ago to provide maximum voltage and minimum self-discharge). However, what happens in the battery, why can’t it be charged at too high a voltage?
Why do you need to add water to a car battery, but you don’t need to add water to a battery from a UPS? These questions allow us to smoothly move into the area of stress of water decomposition. As I wrote above, when charging a battery, electrolysis occurs. However, not all the current is spent on converting PbSO4 into PbO2 and Pb. Part of the current will inevitably be spent on the decomposition of water, which makes up a significant part of the electrolyte:
2H2O = 2H2 + O2
Theoretical calculation gives a voltage value for this reaction of ca. 1.2V. Let me remind you that the voltage on the cell during charging is obviously more than 2V. Fortunately, water begins to actively decompose only above 2V, and in industry, to obtain hydrogen and oxygen from it, the process is carried out at 2.1-2.6V (at elevated temperatures). Be that as it may, here we come to the conclusion that at the end of the battery charging process there will inevitably
the process of decomposition of water in the electrolyte into elements occurs. The resulting oxygen and hydrogen simply evaporate from the reaction sphere. There are the following myths about them:
1.
Hydrogen is extremely explosive! You overcharge the battery and at the very least you will lose the room where it was!
In fact, negligibly little hydrogen is released during the electrolysis process compared to the volume of the room. Hydrogen explodes at a concentration of 4% in air. If we assume that electrolysis is carried out in a room measuring 3*3*3 meters or 27 cubic meters, then we will need to fill the room with 27*0.04=1.1 cubic meters. hydrogen. To obtain such an amount of H2 it would be necessary to completely decompose approx. 49 moles of water or 884 grams of it. If anyone has observed electrolysis, they will understand how much this is. Or let's try to go to time. With a standard charging current for large batteries of 6A, Faraday's equation gives the time required to produce this amount of hydrogen as much as 437 hours or 18.2 days. To fill a room with hydrogen to an explosive concentration, you need to forget about charging for 2 and a half weeks! But even if this happens, the concentration of sulfuric acid will simply increase until its solution acquires too high a resistance for the measly 12V charging and the current becomes negligible. And the hydrogen will simply evaporate.
Explosions very rarely occur directly in the housings of large batteries due to the fact that the released hydrogen for some reason cannot leave the confined space. But even in this case, nothing terrible happens - most often the explosion is only enough to cause a slight deformation of the upper part of the body, but not to break the lead connections. And the battery can still continue to work even after such damage.
2.
Electrolysis can produce deadly poisonous and no less explosive than hydrogen, hydrogen sulfide!
Not ours, periodically I came across a myth in English-language posts. Theoretically, of course, it is possible to apply such a high voltage and create so. such a high current that the process of reduction of sulfate ion will begin at the cathode. The voltage for this will be sufficient, and the reduction products will not have time to diffuse away from the electrode and the reduction will proceed further. But charging within ten to three volts and with a current limit of 6A is hardly capable of this. Once, I observed the process of reducing sulfate to SO2, yes, it is possible; classmates mistakenly did something wrong during the experiment. But this is very rare because... there the concentration of sulfuric acid was noticeably higher than that used in batteries, the design of the electrode and its material were different and, naturally, the voltage and current were exorbitant. And SO2 is not H2S.
3.
During electrolysis, arsenic and antimony from the grating material will be reduced to poisonous arsine and stibine!
Indeed, the gratings contain relatively a lot of antimony; there is probably no arsenic in modern gratings at all. When the battery is operating, the grid on which recovery occurs, i.e. cathode cannot be destroyed. Even if stibine were somehow released, it would immediately interact with PbSO4, reducing it to metal.
However, there is some practical trouble here. Hydrogen and oxygen gases can
carry droplets of electrolyte along with them, creating an aerosol of sulfuric acid. An aerosol of sulfuric acid, even concentrated, is not dangerous for humans and simply causes a cough. However, sulfuric acid is a nightmare for fabrics and paper. If even a small amount of sulfuric acid gets on clothing, holes will certainly appear there or the fabric will tear in that area. In weeks, if there is a lot of acid, in a month, but the clothes will rot.
So you shouldn’t be afraid of gas emissions from a household point of view, or you should, but you need to focus specifically on the aerosol of sulfuric acid.
So, water has begun to decompose into hydrogen and oxygen, there is less and less of it in the electrolyte, what next? If this is a battery in which the electrolyte is simply poured in the form of a layer of liquid, then an increase in self-discharge will begin due to an increase in the concentration of sulfuric acid. It is interesting that this will be accompanied by a slight increase in voltage (the acid concentration increases) on the cell. That is why car owners must constantly monitor the concentration of sulfuric acid in their batteries (using a hydrometer) and add water there. The procedure for adding water is a necessary part of the maintenance process.
any battery. Except for one type, and we'll talk about that now.
Having a battery in which a layer of liquid, caustic towards metals, dangles is of course inconvenient, and therefore attempts to get rid of the liquid directly were made a long time ago, starting almost in the first half of the 20th century. By the way, it’s not like a layer of sulfuric acid was splashing around the electrodes. In reality, it is well distributed between the electrodes and the separators surrounding them, even in cheap models. So the first option was to use fiberglass. Simply surround the electrodes with fiberglass impregnated with sulfuric acid and most problems will be solved. This type of battery is called AGM (absorbent glass mat) and the vast majority of such batteries are for UPS. Although such small form factor batteries are often positioned as those that can be used in any position, we cannot completely agree with this. Opening the cover of a standard cheap AGM battery shows that there are no special covers there, and therefore, only capillary forces keep the electrolyte from leaking out. I’m almost sure that if you drive an AGM battery upside down, then after one charge, sulfuric acid will flow out of it under gas pressure.
The second common type is more interesting, this is the so-called. gel batteries. And they are obtained thanks to the following. If soluble silicates are acidified, silicic acid will be released:
Na2SiO3 + H2SO4 = Na2SO4 + SiO2
+ H2O
If the original silicate solution is of poor quality, the silicic acid will precipitate as a glassy mass, but if it is pure enough, the silicic acid will precipitate as a nice piece of uniform, translucent gel. The method for producing gel batteries is based on this - simply adding silicates to the electrolyte causes it to harden into a gel-like mass. Accordingly, there is nothing left to leak from there and the battery can really be used in any position. The process of gel formation itself does not increase the capacity of the battery or improve its qualities, however, manufacturers use it in the production of the highest quality models, and therefore these batteries are of high quality and greater capacity. It is interesting that in both cases the electrolyte carrier is SiO2 in one form or another.
Both types of batteries are combined into the glorious VRLA type - valve-regulated lead-acid battery, which is used in UPSs. Formally, they are considered maintenance-free and can be used in any position, but this is not entirely true. Moreover, many have already encountered the effect when literally a few ml of water brings back to life a seemingly dead battery from a UPS. This happens because these batteries are not at all immune from electrolysis of water in the electrolyte, and therefore from drying out. Everything happens exactly the same as in large batteries. But the most expensive and cool maintenance-free batteries contain a catalyst for the recombination of released gases back into water, and now their case is truly completely sealed. Please note that AGM and GEL type batteries can be truly sealed and maintenance-free, but they may not be and may not contain a catalyst for the recombination of oxygen and hydrogen. Then, despite the seemingly advanced design, the user will have to either buy new batteries more often or add water using a syringe.
I would like to add a few words about discharge modes. Battery manufacturers indicate the maximum allowable current for a particular model, but you need to understand that a battery is simply a mixture of chemicals and the emf is generated exclusively chemically. This is not a capacitor which, by electrohydraulic analogy, can be compared to some kind of mechanical vessel (with a flexible membrane). Although batteries can produce very high currents, in reality they operate best at low currents, both in discharge and in charge. Therefore, UPSs designed to charge small batteries will charge them in the most gentle mode when working with large ones. However, over the course of more than one day. It is interesting to note that the higher the power of the UPS, the more batteries the manufacturer prefers to assemble in series. Everything is logical here - small batteries withstand high discharge currents very poorly.
To sum it up:
1. Small-sized and large-sized batteries are identical in design.
2. For the vast majority of batteries of any size, adding water is a necessary part of routine maintenance.
3. Only a few of the expensive battery models contain a gas recombination mechanism and can be called truly maintenance-free.
4. The hydrogen itself, which is released during charging (which is equal to constant operation in a UPS) of the battery, is not a significant threat or problem.
5. You need to work very carefully with the battery, carefully avoiding spilling even the slightest drops of electrolyte, or you will lose your clothes.
6. Discharge and charge with low currents are the most preferred modes of battery operation.
Lead-acid battery module
The battery contains the electrical energy necessary to start a truck. The truck has two identical 12-volt batteries that are connected in series and together produce a voltage of 24 V (V-volts). When you turn the ignition key, the batteries supply power to the starter. The starter begins to rotate the engine crankshaft. At the same moment, the generator starts and recharges the batteries.
Batteries also supply electricity to the consumer if the generator does not provide enough power.
On most trucks, lead-acid batteries are mounted to the frame, some on the left, others on the right. The batteries can also be installed under the truck cab.
These additional devices are needed to power other devices in the cabin: a refrigerator, a media player, or a mobile phone charger.
Because the device contains hazardous acids, there are several signs affixed to the device to indicate how to handle it safely.
These signs indicate that:
- The device should be stored in a hard-to-reach place.
- There is a risk of explosion during charging.
- The battery should be kept away from open flames.
- The battery contains dangerous corrosive acid.
- Always wear safety glasses when servicing.
- Before starting work, you must read the operating instructions.
- For disposal, the battery must be handed over to a special collection point.
- The battery should never be thrown away with regular trash as it contains lead and acid.
Each device contains information about what type of battery you are working with. This information meets certain standards.
There are various organizations that deal with standards, for example:
The sticker contains the necessary information. For example,
- 12 V (volt): voltage. This is a 12 V battery.
- 230 A/h (amp-hour): capacity. This is the maximum amount of electrical energy that a battery can produce with a 20-hour discharge (at a final voltage of 10.5 V and a temperature of 25 ℃). This is the maximum amount of electrical energy that the battery can store. The data is indicated taking into account the discharge time of 20 hours.
- 1200 A, cold cranking current. In accordance with the DN standard, this battery is capable of providing 1200 A power for 30 seconds with a voltage of at least 9 V and a temperature of -18 ℃.
- Two identical batteries are connected in series. This means that together they supply a voltage of 2×12V=24 V. The battery capacity is constant and in our example is equal to 230 A/h.
- Both batteries must be of the same brand and have the same capacity and voltage. They must also have the same production date.
Cyclic and buffer operation of the battery
On some batteries, the case shows the maximum charging current and two voltages, or actually two voltage ranges.
The permissible cycle voltage applies to cyclic operation, that is, when the battery is charged for several hours and then discharged for a longer or shorter time. An example would be an electric scooter battery that operates cyclically.
On the other hand, standby mode is the so-called buffer operation, when the battery is always energized and ready for use. A typical example is a UPS. Another example is a backup power supply for alarms. Then the battery is constantly connected to the charger - the power supply, so the voltage is lower than in cyclic mode.
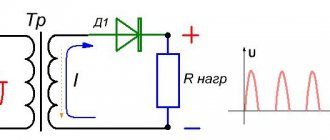
As shown in the photo, the voltage during float mode should be 13.5 - 13.8 V, in cyclic mode the final charge voltage can be 14.4 - 15.0 V. Since a 12 volt battery has six cells, this gives 2 .25 – 2.30 V/cell in buffer mode and 2.4 – 2.5 V/cell in cyclic mode.
Removing batteries
Before you begin removing the battery from the vehicle, you must read the repair manual. Please note that the instructions will differ depending on the brand of battery.
- Remove the key from the ignition.
- Remove the protective cap from the battery casing and terminal covers (they are sometimes attached).
- Disconnect the battery cable clamp from the negative terminal first (if you disconnect the battery cable clamp from the positive terminal first and it accidentally touches the body, it will cause a short circuit.
- Then disconnect the other three battery terminals.
- Disconnect the connecting cable between batteries 1 and 2.
- Then remove both fasteners.
- Remove both batteries from the compartment.
Battery Installation
Before proceeding with installation, please read the repair manual. Each brand has its own instructions.
- Install new batteries.
- Clean the terminals and clamps.
- Install the jumper cable with clamps to battery negative terminal 2 (rear battery) and battery positive terminal 1 (front battery). Pay special attention to the terminals as the plus terminal has a larger diameter than the minus terminal. Both terminals often taper at the top.
- Install the clamp for the positive terminal 2.
- Then install the clamp for negative terminal 1.
- Lubricate the battery terminals and clamps with grease to prevent corrosion.
- Place the protective caps on the pole covers (if equipped).
- Place the protective cover on the battery case.
- Insert the key into the ignition.
Serial and parallel connection
Voltage refers to the potential difference between its positive and negative terminals. voltage is symbolized by the letter U. The unit of voltage is the volt. Voltage is equal to current (A) at a certain resistance (R).
Battery capacity is the maximum amount of electrical energy that a battery can contain. The unit of capacity is ampere-hour/Ah. Thus, by power we mean current (A).
There are also combined circuits in which batteries are connected both in parallel and in series. This increases both the capacitance and the voltage.
Varieties and testing
The screenshots clearly show the modules that characterize different types of SCAB. These are standard solutions (with liquid electrolyte) and devices manufactured using advanced technologies.
Taking tests in LMS ELECTUDE will allow you to figure out how you were able to understand the structure of a lead-acid battery and whether you are ready to solve the problems associated with it.