What is a car battery, purpose
The fact that the battery is responsible for all electrical equipment in the car was indicated above, but not everything is so simple and unambiguous. The main task of the battery is to ensure the start of the power unit.
When the engine is started, the entire on-board network is powered from the generator. In the mid-20th century and even towards the end there were internal combustion engines without batteries, such as motorcycle engines. In them, the launch was carried out due to muscular force, and then all systems worked from the generator.
However, recently, with the saturation of cars with various electrical appliances, multimedia centers or climate systems, generators do not always cope with providing them with energy. In this case, the recharge comes from the battery.
But let's return to the main purpose of the battery. Be that as it may, the main task still remains to provide electricity to the engine starter.
Purpose of a car battery
You are probably familiar with the abbreviation AKB, but not everyone knows what the letter “K” stands for. So, this letter combination stands for “car acid battery,” and although modern batteries are not all acid batteries, in relation to cars this is the predominant type of battery.
Car battery functions:
- power supply to the starter to start the internal combustion engine. The battery is capable of providing electricity to the starter for 30 seconds. The latter, being an electric motor, performs a cold start of the main power unit;
- the battery can provide power to the vehicle’s on-board network if the load on the generator exceeds its ability to supply power to consumers;
- When the engine is not running, the on-board network is powered exclusively by the battery.
Note that using a battery as a power source while the engine is running is an extraordinary situation, indicating either too many consumers (or rather, their total power consumption), or serious problems in the on-board network that require diagnosis and prompt elimination.
However, for some time before the generator reaches its optimal operating mode (this happens when the crankshaft speed reaches about 1800 rpm), part of its functions are performed by the battery. After the generator operates at full power, it begins to replenish the battery capacity lost when starting the engine and continues to do this until the voltage at the battery contacts increases to a certain value.
Since the active substance of the battery is an acid solution, it requires careful handling. Acid vapors released when the electrolyte boils (a process typical when charging a battery using a charger) can also pose a danger. Maintenance-free batteries are less dangerous in this regard, since gas emissions to the outside are minimized.
What is it made of and what's inside the battery?
Despite all the technological progress, cars still use batteries invented in the mid-19th century.
Gaston Plante is considered the inventor of the battery, who invented it in 1860. Well, the battery acquired its modern appearance in 1878, after it was improved by Camille Faure.
Since that time, the batteries have not changed fundamentally, all changes were only cosmetic, relating to their appearance and the quality of manufacturing of structural elements.
These batteries are called lead-acid, and the name contains a description of the operating principle of these devices.
A 19th century drawing showing a cross-section of one of the first batteries.
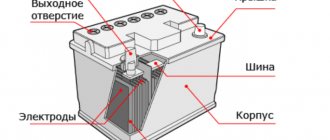
So, the battery consists of the following main parts:
- Housings;
- Lids;
- Negative electrodes;
- Positive electrodes;
- Positive terminal;
- Negative terminal;
- Connecting jumpers;
- Filler plugs;
- Electrolyte
Next, we will consider each design element.
So, the battery case and cover consists of acid-neutral plastic.
The negative plates, like the positive ones, consist of metal lead and are made in the form of a lattice.
In the negative plate, the interstices of the lead grid are filled with metallic lead, in the form of a compressed powder. In the positive case - compressed lead dioxide powder (PbO2).
In the space between the plates there are separators, which are microporous plates made of ebonite or revertex. Both materials can be considered a kind of rubber, and they are made from rubber.
The purpose of the separators is to separate the positive and negative electrodes and prevent them from short-circuiting, which can occur as a result of vibrations from the engine and the entire vehicle.
Both terminals are made of metal lead and through them the battery is connected to the on-board network of the machine.
Li-po and technology
A laptop equipped with a lithium-ion battery can support 3 times more charge cycles (that is, 3 times longer) than a laptop with a standard lithium-ion battery.
Energy efficiency is achieved not only through battery chemistry. Leaving the laptop connected to the charger when the battery is already fully charged may degrade battery performance and thus shorten its life. This can also cause the battery to swell due to internal gas build-up caused by oxidation, resulting in deformation or damage to the laptop. Additional software technologies allow you to set the charge limit to 60%, 80% or 100% to extend battery life and reduce the likelihood of battery swelling.
Laptops are also equipped with a fast charging mechanism, with which the battery is charged to just over half in a few tens of minutes.
The principle of operation of the battery
The operating principle of the battery is based on the electrochemical reaction of lead oxidation in a solution of sulfuric acid and water.
When the battery is discharged, metallic lead oxidizes on the positive plate, while lead dioxide is reduced on the negative plate.
When charging, the reverse process occurs, the amount of lead dioxide on the negative plate decreases, and the amount of metal on the positive plate increases.
Also, when the battery is discharged, the amount of sulfuric acid in the electrolyte decreases and the amount of water increases. When charging, the reverse process also occurs.
TOP battery rating
Many brands, many advice, difficult choices - these are the problems buyers face. We offer a small, our, subjective rating of battery brands.
- The first place in terms of quality and durability is rightfully occupied by OEM batteries. OEM is an analogue of a part that was installed from the factory. Of course, you will have to pay a lot more for a battery that proudly displays the Mercedes or Honda logo than for any other brand, but the result is worth it. The most popular battery brands on the market are Varta and Bosch. They have earned a reputation as reliable, trouble-free batteries that faithfully work out every penny invested.
- Among those who like to pay less and get more, the Topla brand is especially valued. This is, of course, not Bosch, but it may well please you with its long service life.
- And our hit parade is completed by budget brands Sada, Styer, Bi-Power and Ista. Although they are not expensive, they are quite capable of providing stable performance. You can remember them when you need a battery urgently, but you don’t have enough money.
Design features of modern batteries
Despite the fact that, in principle, batteries have not changed for more than 150 years, modernity has made serious changes in the technology of their manufacture and in the materials from which they are made.
Let's look at them separately:
- Plates
Today, on the highest quality batteries, the plate material has undergone slight changes. Now plates are made not from pure lead, but from its alloy with silver. At the same time, it became possible to reduce the weight of the battery by a third, and increase its service life by 20%.
In addition, the technology of their production itself has changed. If the first plates were produced by casting them, today they are made from a thin lead sheet by stamping. This method is cheaper and the plates are stronger and thinner.
- Separators
One of the reasons for battery failure is a short circuit of the positive and negative plates.
The short circuit occurs due to the fact that the active zone crumbles from the plates and it shorts out at the bottom of the cans. To avoid this, separators are made in the form of envelopes sealed from below, under the plates. Thus, when the active zone crumbles, it remains inside the envelope and does not close.
Today, glass fiber is added to the material of the separators themselves. This also allows you to make them thinner and stronger.
- Electrolyte
As stated above, the electrolyte is a solution of sulfuric acid and water. Under the influence of low temperatures, as is known, water freezes, but this does not happen with the electrolyte.
But it still noticeably thickens and loses its properties, which is why the battery capacity is noticeably reduced. To avoid this, today various additives are added to the electrolyte.
- Gel electrolytes
Batteries with gel electrolytes can be considered the pinnacle of the evolution of acid batteries and that is why a separate section is dedicated to them. Such batteries are simply called gel batteries. In these devices, the electrolyte is so modified that it becomes something like jelly.
This modification, in combination with the other innovations described above, gave truly magical results. The batteries have become practically eternal, immune to overturning, practically do not lose their properties in winter, and at the same time are much lighter in weight.
Characteristics of a maintenance-free battery
Today's level of technical development has made it possible for automakers to use the most advanced and high-quality batteries - maintenance-free batteries.
The design of a maintenance-free car battery has characteristic features that give consumers a pleasant opportunity to pay a minimum of attention to this battery.
Car Battery Maintenance
It is worth noting that a maintenance-free battery is a modern source of energy, which in its design does not require or have special holes for adding water or electrolyte; the case of these batteries is completely sealed.
More than 150 years have passed since the development of the car battery and its basic structure remains unchanged for any type of battery to this day. The main elements of the battery are: acid and lead plates.
Battery parameters and characteristics
The parameters and characteristics of batteries are encrypted in their markings and now we will figure out what it means.
We will consider this issue using the example of the most common battery 6ST-55.
So, in the name of the battery, the number 6 means that the battery consists of 6 cans.
- ST – means that the battery is a starter one.
- 55 – indicates the battery capacity, which is 55 Amp*hour.
In order to understand what kind of battery you need, you need to know two parameters:
- Engine type;
- Engine size of your car;
Next, we will consider which engines and which batteries are suitable. This is the table for gasoline engines:
- Engines up to 1.6 liters. Batteries 6ST-45 are suitable for them;
- Engines with a volume of 1.6 to 2.5 liters. 6ST-55 is suitable for them;
- Engines with a volume of 2.5 to 3 liters. 6ST-60 is suitable for them;
- Engines with a volume of 3 to 3.5 liters. 6ST-75 is suitable for them;
- Engines with a volume of more than 3.5 liters. 6ST-90 is suitable for them.
For diesel power units, these parameters are slightly different:
- Engines up to 1.5 liters. 6ST-55 is suitable for them;
- Engines with a volume of 1.5 to 2.0 liters. 6ST-60 is suitable for them;
- Engines with volume from 2 to 2.7 liters. 6ST-75 is suitable for them;
- Engines with a volume of 2.7 to 3.5 liters. 6ST-90 is suitable for them;
- Engines with volume from 3.5 to 6.5 liters. 6ST-132 is suitable for them;
- Engines with a volume of more than 6.5 liters. 6ST-192 and more are suitable for them.
As you can see, due to the different operating principles of diesel and gasoline engines, batteries of different capacities are used for them.
For diesel powertrains, you will need larger batteries.
Rechargeable batteries
To ensure the normal functioning of equipment, batteries of different sizes are used. The main area of their use is powering small household devices.
Rechargeable batteries are used for a wide variety of devices - radio mice, keyboards, cameras, simple flashlights, watches, and other small electronics.
They have different sizes:
- AA (finger) - the most common format of round batteries with a length of 5 cm, a voltage of 1.2 V and a capacity of 1000-3000 mAh
- AAA (mini-finger) - also widespread, have a length of 4.4 cm, the same voltage of 1.2 V, but a smaller capacity of 500-1500 mAh
- crown - a rarer rectangular battery with a voltage of 9 V, used in some electrical appliances (for example, multimeters)
There are other, rarer battery formats:
- CS (Sub C) – short round battery
- C (R14) – medium coin cell battery
- D (R20) – large round battery
They are not very common and are used in some specific devices and old cameras.
The best popular manufacturers of rechargeable batteries include Panasonic, Varta, Ansmann, Sanyo. There are also many other famous brands, but they are more often counterfeited.
Battery low
The design of such power supplies requires the presence of two terminals: plus and minus. Their operation occurs in this way: when there is no load, the electrical circuit is open, and when connected to the poles of any device, the circuit is closed and the battery begins to discharge. The discharge current flowing through the battery under such conditions occurs due to the movement of ions between the electrodes: anions and cations.
It is most convenient to consider the process of discharging the battery in more detail using a specific example. The cathode (positive electrode in the current source) consists of nickel oxide hydrate, to which graphite powder is added to improve conductivity.
To make the anode (negative electrode) in batteries of this type, iron grids with cadmium sponge are used. The electrolyte in such a device will be a mixture of potassium and lithium hydroxides. Nickel oxide-hydroxide in such an alkaline power source enters into a chemical interaction with cadmium atoms and water molecules. As a result of this reaction, cadmium and lithium hydroxides are formed, and electricity is also released.
Tips for battery operation and maintenance
In order for the battery to last as long as possible, you need to pay very little attention to it. Here are some tips for operating a car battery.
- Deep discharge is the enemy of the battery. Every time the battery is discharged to zero, irreversible sulfation of the electrodes occurs, calcium batteries especially suffer from this. From time to time, it is advisable to fully charge the battery with a special charger and under no circumstances allow it to completely discharge.
- The second enemy is vibration. Due to strong shaking and regular impacts, the active layer falls off the plates. AGM batteries suffer less from this, liquid batteries suffer more.
- Battery terminals are prone to oxidation, which impairs contact. Periodically you need to pay attention to the condition of the terminals and, if necessary, clean them of oxides.
- Pay attention to the battery case. Dirt, oil, and moisture contribute to current leakage and self-discharge.
- Electrical problems can also damage the battery. Especially problems with the starter and generator - adjacent elements.
- A swollen case with traces of electrolyte indicates that it is time to buy a new battery. A damaged battery must not be used!
Sources
- https://www.calc.ru/Ustroystvo-Akkumulyatora.html
- https://akbinfo.ru/ustrojstvo/ustroistvo-avtomobilnogo-akkumuljatora.html
- https://TechAutoPort.ru/elektrooborudovanie-i-elektronika/istochniki-pitaniya/akkumulyator.html
- https://remam.ru/elektrik/printsip-raboty-i-ustrojstvo-akkumulyatora-avtomobilya.html
- https://promercedes.ru/akkumulyator/iz-chego-sostoit-avto-akkumulyator
- https://principraboty.ru/princip-raboty-akkumulyatora/
- https://VazNeTaz.ru/akb-avtomobilnyi-akkumulyator
- https://ProAkkym.ru/obzor/ustrojstvo-akkumuljatora
- https://www.akbvolt.ru/articles/kak-ustroen-akb-obschee-opisanie.-tipy-korpusov-azija-evropa-amerika/
- https://AutoVogdenie.ru/avtomobilnyj-akkumulyator-chto-eto-ustrojstvo-i-princip-raboty-akb.html
- https://tolkavto.ru/remont-i-obsluzhivanie/elektrooborudovanie/printsip-raboty-akkumulyatora.html
- https://tze1.ru/articles/detail/svintsovo-kislotnye-akkumulyatory/
Electrode device
A lead-acid battery can be used as an example. Each cell of such a battery contains a pair of electrodes and separating plates, which are made of porous material that does not react chemically with acid. Such plates are designed to prevent short circuits of electrodes immersed in the electrolyte, and are called separators.
We recommend: Measuring the internal resistance of a car battery
The electrodes in such batteries are made in the form of flat lead grids. Powdered lead dioxide (in anode plates) and metal lead in powder form (in cathode plates) are pressed into the cells of such gratings. The use of powders is driven by the desire to increase the interface area at the electrolyte-electrode interface, which significantly increases the capacity of such a current source.
There are experimental samples of batteries in which lead grids are replaced with electrodes consisting of woven carbon fiber threads, which are coated with the finest lead coating. This technology allows you to use significantly less lead due to its distribution over a large area, which makes the battery not only smaller and lighter, but also increases its efficiency. The efficiency is higher than traditional ones, and the charging time is greatly reduced.