Silicon crystal lattice
In the normal state, silicon atoms form a crystal lattice
.
There are four electrons in the outer electron shell of an atom. With their help, a covalent bond
with four neighboring atoms. Each electron in such a bond belongs to two atoms at the same time. Thus, each atom has eight electrons in its outer electron shell. As a result, since the last level of the electron shell is complete, it is very difficult to remove its electrons from the atom and the material behaves like a dielectric (does not conduct electric current).
INFOFIZ
According to the value of electrical resistivity, semiconductors
occupy an intermediate place between conductors and dielectrics. Semiconductors include many chemical elements (germanium, silicon, selenium, tellurium, arsenic, etc.), a huge number of alloys and chemical compounds.
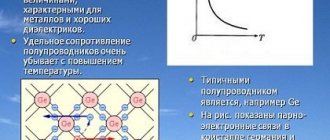
The qualitative difference between semiconductors and metals is manifested primarily in the dependence of resistivity on temperature. As the temperature decreases, the resistance of metals decreases. In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators.
Dependence of resistivity ρ of a pure semiconductor on absolute temperature T
.
Semiconductors
are substances whose resistivity decreases with increasing temperature.
Such a course of the dependence ρ( T
) shows that in semiconductors the concentration of free charge carriers does not remain constant, but increases with increasing temperature. The mechanism of electric current in semiconductors cannot be explained within the framework of the free electron gas model. An explanation of the phenomena observed in conductors is possible on the basis of the laws of quantum mechanics. Let us qualitatively consider the mechanism of electric current in semiconductors using germanium (Ge) as an example.
Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons
.
In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent
, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms.
The valence electrons in a germanium crystal are much more strongly bound to the atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current. As the temperature increases, some of the valence electrons may gain enough energy to break covalent bonds. Then free electrons
(conduction electrons). At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons.
Vacancies that are not occupied by electrons are called
holes
.
The vacant place can be occupied by a valence electron from a neighboring pair, then the hole moves to a new place in the crystal. electron-hole pairs are formed per unit time
.
At the same time, the reverse process occurs - when a free electron meets a hole, the electronic bond between the germanium atoms is restored. This process is called recombination
.
Recombination is
the restoration of electronic bonds between atoms.
Electron-hole pairs can also be created when a semiconductor is illuminated due to the energy of electromagnetic radiation.
In the absence of an electric field, conduction electrons and holes participate in chaotic thermal motion.
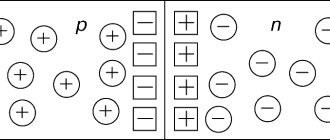
If a semiconductor is placed in an electric field, then not only free electrons are involved in the ordered movement, but also holes, which behave like positively charged particles. Therefore, the current I
in a semiconductor it consists of electron
In
and hole
Ip
currents:
I
=
In
+
Ip
Electric current in semiconductors
is the directional movement of electrons to the positive pole, and holes to the negative.
The concentration of conduction electrons in a semiconductor is equal to the concentration of holes: nn
=
np
.
The electron-hole conductivity mechanism appears only in pure (that is, without impurities) semiconductors. It is called the intrinsic electrical conductivity
of semiconductors.
The intrinsic electrical conductivity
of semiconductors is called the electron-hole conduction mechanism, which manifests itself only in pure (that is, without impurities) semiconductors.
In the presence of impurities, the electrical conductivity of semiconductors changes greatly.
Impurity conductivity
is the conductivity of semiconductors in the presence of impurities.
A necessary condition for a sharp decrease in the resistivity of a semiconductor upon the introduction of impurities is the difference in the valence of the impurity atoms from the valence of the main atoms of the crystal.
There are two types of impurity conductivity - electronic
and
hole
conductivity.
- Electronic conductivity
occurs when an impurity with a higher valency is introduced into a semiconductor crystal.
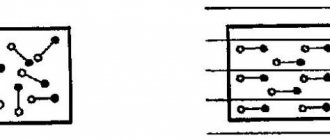
For example, pentavalent arsenic atoms, As, are introduced into a germanium crystal with tetravalent atoms.
The figure shows a pentavalent arsenic atom found in a site of the germanium crystal lattice. The four valence electrons of the arsenic atom are included in the formation of covalent bonds with four neighboring germanium atoms. The fifth valence electron turned out to be extra; it easily breaks away from the arsenic atom and becomes free. An atom that has lost an electron becomes a positive ion located at a site in the crystal lattice.
Donor impurity
is an impurity of atoms with a valence exceeding the valence of the main atoms of the semiconductor crystal.
As a result of its introduction, a significant number of free electrons appear in the crystal. This leads to a sharp decrease in the resistivity of the semiconductor - thousands and even millions of times. The resistivity of a conductor with a high content of impurities may approach that of a metal conductor.
In a germanium crystal with an admixture of arsenic, there are electrons and holes responsible for the crystal’s own conductivity. But the main type of free charge carriers are electrons detached from arsenic atoms. In such a crystal nn
>>
n.p.
.
Conduction in which the main carriers of free charge are electrons is called
electronic conductivity.
A semiconductor that exhibits electronic conductivity is called
an n-type semiconductor
.
- Hole conduction
occurs when an impurity with a lower valence is introduced into a semiconductor crystal.
For example, trivalent In atoms are introduced into a germanium crystal.
The figure shows an indium atom that has created covalent bonds with only three neighboring germanium atoms using its valence electrons. The indium atom does not have an electron to form a bond with the fourth germanium atom. This missing electron can be captured by the indium atom from the covalent bond of neighboring germanium atoms. In this case, the indium atom turns into a negative ion located at a site of the crystal lattice, and a vacancy is formed in the covalent bond of neighboring atoms.
Acceptor impurity
is an impurity of atoms with a valence lower than the valence of the main atoms of a semiconductor crystal capable of capturing electrons.
As a result of the introduction of an acceptor impurity, many covalent bonds are broken in the crystal and vacancies (holes) are formed. Electrons from neighboring covalent bonds can jump to these places, which leads to chaotic wandering of holes throughout the crystal.
The presence of an acceptor impurity sharply reduces the resistivity of the semiconductor due to the appearance of a large number of free holes. The concentration of holes in a semiconductor with an acceptor impurity significantly exceeds the concentration of electrons that arose due to the mechanism of the semiconductor’s own electrical conductivity: np
>>
nn
.
Conduction in which the main free charge carriers are holes is called
hole conductivity
.
A hole-conducting semiconductor is called
a p-type semiconductor
.
It should be emphasized that hole conductivity is actually caused by the movement of electrons through vacancies from one germanium atom to another, which carry out a covalent bond.
Dependence of electrical conductivity of semiconductors on temperature and illumination
- In semiconductors,
the mobility of electrons and holes decreases with increasing temperature, but this does not play a noticeable role, since when the semiconductor is heated, the kinetic
energy of the valence electrons increases and individual bonds break, which leads to an increase in the number of free electrons, i.e., an increase in electrical conductivity
.
- When a semiconductor is illuminated, additional carriers appear in it, which leads to an increase in its electrical conductivity.
This occurs as a result of light stripping electrons from the atom and simultaneously increasing the number of electrons and holes.
Read about what processes occur when p-n-type semiconductors come into contact and where semiconductors are used in the continuation of lecture 32 “Semiconductor diode.” Semiconductor devices»
Types of semiconductor conductivity
Electronic conductivity
Let's add a pentavalent arsenic atom (As) to a silicon semiconductor. Through four valence electrons, arsenic will establish covalent bonds with four neighboring silicon atoms. There will be no pair left for the fifth valence electron and it will become weakly bound to the atom.
Under the influence of an electromagnetic field, such an electron is easily torn off and is drawn into the ordered movement of charged particles (electric current). An atom that has lost an electron turns into a positively charged ion with a free vacancy - a hole.
Despite the presence of holes in a silicon semiconductor with an admixture of arsenic, the main carriers of free charge are electrons. Such conductivity is called electronic, and a semiconductor with electronic conductivity is called an N-type semiconductor.
Hole conductivity
Let us introduce a trivalent indium atom (In) into the silicon crystal. Indium will establish covalent bonds with only three neighboring silicon atoms. For the fourth “neighbor”, indium is missing one electron. This missing electron can be captured by an indium atom from the covalent bond of neighboring silicon atoms.
The indium atom turns into a negatively charged ion, and a vacancy (hole) is formed in the covalent bond of neighboring atoms. In turn, an electron from a neighboring covalent bond can jump to this place. The result is a chaotic wandering of holes throughout the crystal.
If you place a semiconductor in an electromagnetic field, the movement of holes will become ordered, i.e. an electric current will occur. Thus, hole conductivity is ensured. A hole-conducting semiconductor is called a P-type semiconductor.
Types and properties of semiconductors
In order for an electric current to appear, there must be mobile particles that carry charges. The electrical conductivity of a substance depends on the number of such carriers per unit volume. In dielectrics they are practically absent, and in semiconductors free carriers are present only in small quantities. Consequently, the resistivity of semiconductors is very high, and in dielectrics it is even higher. There are different types of these materials, each with their own specific properties.
All semiconductors can be divided into several main types. Among them, the leaders are pure or proprietary materials, which do not contain any impurities.
They are characterized by a crystalline structure, where the atoms are arranged in a periodic order at its nodes. Here there is a stable mutual connection between each atom and four atoms located nearby. This makes it possible to form permanent electron shells, which contain eight electrons. At a temperature equal to absolute zero, such a semiconductor becomes an insulator, since all electrons are connected by covalent bonds.
When the temperature increases or any irradiation occurs, electrons can escape from covalent bonds and become free charge carriers. When moving, the free spaces are gradually occupied by other electrons, so the electric current flows only in one direction.
In electronic semiconductors, in addition to the four atoms that form the basis of the crystal lattice, there are so-called donors. They are impurities in the form of pentavalent atoms. The electron contained in such an atom cannot normally enter into a covalent bond and is therefore separated from the donor. Thus, it turns into a free charge carrier. In turn, the donor becomes a positive ion, this can happen even at room temperature.