The task of dielectric physics is, first of all, to establish patterns and create a theory of interaction between the electromagnetic field and matter, on the basis of which it is possible not only to explain the phenomena of this interaction, but also to predict the properties and behavior of dielectrics when changing state parameters, as well as to develop various materials with predetermined properties .
Physically, this task comes down to the theoretical and experimental establishment of a connection between the chemical composition, state of aggregation, structure of dielectrics and their macroscopic properties.
The term “dielectric” was first introduced by M. Faraday in 1837 to characterize substances into which an electric (electromagnetic) field penetrates. Most often, dielectrics are understood as substances through which, ideally, electric current does not pass, but in practice does not pass well.
This is due to the internal structure of atoms and molecules of dielectrics and, above all, their lack of charges that could move freely over macroscopic distances under the influence of a field.
Electrical dielectrics. What are they?
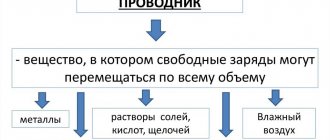
As we were taught in school, some substances conduct electricity poorly, while others conduct electricity well. For example, wood conducts very poorly, but aluminum conducts much better. So, if you remember the terminology, then substances that conduct electricity well are called conductors, and those that conduct it poorly are called... Well, what about them? Oh yes, they are called electrical dielectrics.
Of course, we are not saying that they do not conduct current at all, no. They are, of course, conductors, just comparatively rather poor ones. Dielectrics, on the other hand, are also substances that can store an electric field within themselves for quite a long time, and this will not require external energy.
Electrical strength.
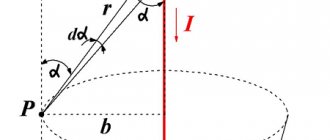
An increase in air pressure leads to an increase in the corona discharge voltage and the electric field strength at which breakdown occurs for the electrode system under consideration. According to Paschen's law, in a uniform electric field the breakdown voltage will not change if, while reducing the interelectrode gap, the gas pressure in the gap is increased by the same amount. Common gases such as nitrogen, oxygen and carbon dioxide have an insulating ability similar to that of air at atmospheric pressure. Some vapors, especially those containing sulfur, chlorine or fluorine, such as sulfur hexafluoride (SF6), carbon tetrachloride (CCl4) and Freon-12 (CCl2F2), have three times the dielectric strength of air at the same pressure. The effect of pressure on breakdown voltage for some materials is shown in the figure.
Also on topic:
ELECTROMAGNETIC RADIATION
The electrical insulating properties of gases are worst at pressures from 1 to 0.01 kPa. The passage of current through a gas at such pressures is accompanied by a bright glow (for example, in mercury or neon lamps). This phenomenon is called a glow discharge.
What happens if you influence from the outside?
If you apply an external electric field to an electric dielectric, then the free charges of the dielectric will begin to gradually neutralize it. Moreover, this will happen until the electrons run out or the resulting field becomes zero.
To understand what substances can generally interact with electric fields, we need to understand a term such as electrical conductivity. In simple terms, in order to interact with an electric field, a substance must have a fairly low electrical conductivity.
To be more precise, the resistivity should be comparable to 1010 Q-cm or even greatly exceed this value.
Where does low electrical conductivity come from?
As we know from the basic physics curriculum, all substances are made of atoms. And these atoms interact very actively with each other. Each of them has its own charge, and thanks to the charges, the atoms interact in one way or another.
However, how is such low electrical conductivity created? It seems that there are atoms, they somehow interact there and current could flow through them, but not everything is so simple. The key to ensuring that the conductivity of a substance is low is a very important fact.
If, when a field is applied, electrons, ions and other particles cannot move freely or do so very poorly, then the electrical conductivity will be low, because everything will stand in its place and free electrons will simply have nowhere to go.
The crystal grid will help you figure it out
Now the crystal lattice will help us understand electrical dielectrics. So that the terms do not seem incomprehensible to us, let's refresh them in our heads. A crystal lattice is a group of points that are formed in substances (more precisely, in crystals) under the influence of shifts (they, by the way, can occur due to the influence of an electric field. Great, we remembered. Now let's figure it out.
As we remember, in an atom that is currently isolated, the energy of electrons cannot take on any values. In this state, the energy will take on clearly designated values W1, W2, W3, etc. Here, take a look at the graph:
Of course, each of these levels will be slightly shifted after the atoms become part of the solid crystal lattice. As a result, the zone in which all energy will be concentrated will be common to the entire lattice.
So, in a crystal lattice, the energy of electrons lies within well-defined zones and all values that are outside this zone are prohibited. We understood this. Let's move on. According to the Pauli principle, each band can accommodate a limited number of electrons. First, electrons will fill the lower levels, and when these rows are completely filled, they will fill the upper rows.
And now the key idea that you need to understand in order to understand why certain substances conduct electric current. Since electrons gradually fill the rows from bottom to top, then at the topmost row they will either fill this row completely or only partially.
So, when the row is partially filled, electrons will be able to move freely along it, which means they will conduct current. Bingo! But if electrons do fill the upper level, then under the influence of an electric field no shifts will occur and, accordingly, such a substance can be called a dielectric.
A very similar situation occurs with amorphous solids (for example, amber or polyethylene). By definition, in such substances the arrangement of atoms is very random, and zones common to the entire crystal simply cannot exist, which means they are also electrical dielectrics.
Links
- Electrical insulating materials (dielectrics)
- Characteristics of electrical insulating materials
What would you like to improve this article?:
|
Dielectric • Semiconductor • Conductor • Superconductor
Dielectrics are substances that do not conduct or conduct electricity poorly. Charge carriers in a dielectric have a density of no more than 108 pieces per cubic centimeter. One of the main properties of such materials is the ability to polarize in an electric field.
The parameter characterizing dielectrics is called dielectric constant, which can have dispersion. Dielectrics include chemically pure water, air, plastics, resins, glass, and various gases.
Properties of dielectrics
If substances had their own heraldry, then the coat of arms of Rochelle salt would certainly be decorated with grapevines, a hysteresis loop, and the symbolism of many branches of modern science and technology.
The pedigree of Rochelle salt dates back to 1672. When the French pharmacist Pierre Segnet first obtained colorless crystals from grapevines and used them for medicinal purposes.
At that time it was still impossible to imagine that these crystals had amazing properties. These properties gave us the right to distinguish special groups from a huge number of dielectrics:
- Piezoelectrics.
- Pyroelectrics.
- Ferroelectrics.
Since the time of Faraday, it has been known that dielectric materials are polarized in an external electric field. In this case, each elementary cell has an electric moment similar to an electric dipole. And the total dipole moment per unit volume determines the polarization vector.
In conventional dielectrics, polarization uniquely and linearly depends on the magnitude of the external electric field. Therefore, the dielectric susceptibility of almost all dielectrics is constant.
P/E=X=const
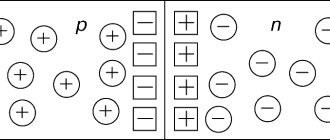
The crystal lattices of most dielectrics are made up of positive and negative ions. Of the crystalline substances, crystals with a cubic lattice have the highest symmetry. Under the influence of an external electric field, the crystal is polarized and its symmetry decreases. When the external field disappears, the crystal restores its symmetry.
In some crystals, electric polarization can arise spontaneously in the absence of an external field. This is what a gadolinium molybdenate crystal looks like in polarized light. Typically, spontaneous polarization is non-uniform. The crystal is divided into domains - areas with uniform polarization. The development of a multidomain structure reduces the total polarization.
Pyroelectrics
In pyroelectrics, spontaneous polarization screens with free charges that cancel out bound charges. Heating a pyroelectric changes its polarization. At the melting temperature, the pyroelectric properties disappear completely.
Some pyroelectrics are classified as ferroelectrics. Their polarization direction can be changed by an external electric field.
There is a hysteresis relationship between the polarization orientation of the ferroelectric and the magnitude of the external field.
In sufficiently weak fields, polarization depends linearly on the field strength. With its further increase, all domains are oriented in the direction of the field, entering the saturation mode. When the field is reduced to zero, the crystal remains polarized. The CO segment is called residual polarization.
The field at which the direction of polarization changes, the segment DO is called coercive force.
Finally, the crystal completely reverses the direction of polarization. With the next change in the field, the polarization curve closes.
However, the ferroelectric state of a crystal exists only in a certain temperature range. In particular, Rochelle salt has two Curie points: -18 and +24 degrees, at which second-order phase transitions occur.
Ferroelectric groups
The microscopic theory of phase transitions divides ferroelectrics into two groups.
First group
Barium titanate belongs to the first group, and as it is also called, the group of bias-type ferroelectrics. In its non-polar state, barium titanate has cubic symmetry.
During a phase transition to the polar state, the ionic sublattices are displaced and the symmetry of the crystal structure decreases.
Second group
The second group includes crystals of the sodium nitrate type, in which the nonpolar phase has a disordered sublattice of structural elements. Here, the phase transition to the polar state is associated with ordering of the crystal structure.
Moreover, in different crystals there may be two or more probable equilibrium positions. There are crystals in which the dipole chains have antiparallel orientations. The total dipole moment of such crystals is zero. Such crystals are called antiferroelectrics.
In them, the polarization dependence is linear, up to the critical field value.
A further increase in the field strength is accompanied by a transition to the ferroelectric phase.
Third group
There is another group of crystals - ferroelectrics.
The orientation of their dipole moments is such that in one direction they have the properties of antiferroelectrics, and in the other direction, ferroelectrics. Phase transitions in ferroelectrics are of two types.
During a second-order phase transition at the Curie point, spontaneous polarization smoothly decreases to zero, and the dielectric susceptibility, changing sharply, reaches enormous values.
During a first-order phase transition, polarization disappears abruptly. Electrical susceptibility also changes abruptly.
The large dielectric constant and electrical polarization of ferroelectrics make them promising materials for modern technology. For example, the nonlinear properties of transparent ferroelectric ceramics are already widely used. The brighter the light, the more it is absorbed by special glasses.
This is effective in protecting the vision of workers in certain industries that involve sudden and intense flashes of light. Ferroelectric crystals with an electro-optical effect are used to transmit information using a laser beam. Within line of sight, the laser beam is simulated in the crystal. Then the beam enters a complex of receiving equipment, where the information is isolated and reproduced.
Piezoelectric effect
In 1880, the Curie brothers discovered that during the deformation of Rochelle salt, polarization charges appeared on its surface. This phenomenon was called the direct piezoelectric effect.
If a crystal is exposed to an external electric field, it begins to deform, that is, a reverse piezoelectric effect occurs.
However, these changes are not observed in crystals that have a center of symmetry, for example, in lead sulfide.
If such a crystal is exposed to an external electric field, the sublattices of negative and positive ions will shift in opposite directions. This leads to polarization of the crystals.
In this case, we observe electrostriction, in which the deformation is proportional to the square of the electric field. Therefore, electrostriction is classified as an even effect.
ΔX1=ΔX2
If such a crystal is stretched or compressed, then the electric moments of the positive dipoles will be equal in magnitude to the electric moments of the negative dipoles. That is, the polarization of the dielectric does not change, and the piezoelectric effect does not occur.
In crystals with low symmetry, during deformation, additional forces of the inverse piezoelectric effect appear, counteracting external influences.
Thus, in a crystal that does not have a center of symmetry in the charge distribution, the magnitude and direction of the displacement vector depends on the magnitude and direction of the external field.
Thanks to this, it is possible to carry out various types of deformation of piezocrystals. By gluing piezoelectric plates, you can get an element that works in compression.
In this design, the piezoelectric plate bends.
Piezoceramics
If an alternating field is applied to such a piezoelectric element, elastic vibrations will be excited in it and acoustic waves will arise. Piezoceramics are used to manufacture piezoelectric products. It represents polycrystals of ferroelectric compounds or solid solutions based on them. By changing the composition of components and geometric shapes of ceramics, its piezoelectric parameters can be controlled.
Direct and inverse piezoelectric effects are used in a variety of electronic equipment. Many units of electroacoustic, radioelectronic and measuring equipment: waveguides, resonators, frequency multipliers, microcircuits, filters operate using the properties of piezoceramics.
Piezoelectric motors
The active element of a piezoelectric motor is a piezoelectric element.
During one period of oscillation of the alternating electric field source, it stretches and interacts with the rotor, and in another it returns to its original position.
Excellent electrical and mechanical characteristics allow the piezo motor to successfully compete with conventional electric micromachines.
Piezoelectric transformers
The principle of their operation is also based on the use of the properties of piezoceramics. Under the influence of the input voltage, a reverse piezoelectric effect occurs in the exciter.
The deformation wave is transmitted to the generator section, where, due to the direct piezoelectric effect, the polarization of the dielectric changes, which leads to a change in the output voltage.
Since in a piezotransformer the input and output are galvanically isolated, the functionality of converting the input signal in voltage and current, matching it with the load at the input and output, is better than that of conventional transformers.
Research into various phenomena of ferroelectricity and piezoelectricity continues. There is no doubt that in the future there will be devices based on new and surprising physical effects in solids.
Classification of dielectrics
Depending on various factors, they exhibit their insulating properties differently, which determine their scope of use. The diagram below shows the structure of the classification of dielectrics.
Dielectrics consisting of inorganic and organic elements have become popular in the national economy.
Inorganic materials are compounds of carbon with various elements. Carbon has a high ability to form chemical compounds.
Mineral dielectrics
This type of dielectrics appeared with the development of the electrical industry. The technology for the production of mineral dielectrics and their types has been significantly improved. Therefore, such materials are already replacing chemical and natural dielectrics.
Mineral dielectric materials include:
•Glass (capacitors, lamps) is an amorphous material, consisting of a system of complex oxides: silicon, calcium, aluminum. They improve the dielectric properties of the material. • Glass enamel – applied to a metal surface. • Fiberglass – strands of glass from which glass fabrics are made. • Light guides – light-conducting fiberglass, a bundle of fibers. • Sitalls are crystalline silicates. • Ceramics – porcelain, soapstone. • Mica – micalex, mica plastic, micanite. • Asbestos is a mineral with a fibrous structure.
Various dielectrics do not always replace each other. Their scope of application depends on cost, ease of use, and properties. In addition to insulating properties, dielectrics are subject to thermal and mechanical requirements.
Liquid dielectrics
Petroleum oils
Transformer oil is poured into power types of transformers. It is most popular in electrical engineering.
Cable oils are used in the manufacture of electrical cables. They impregnate the paper insulation of cables. This increases electrical strength and dissipates heat.
Synthetic liquid dielectrics
To impregnate capacitors, a liquid dielectric is needed to increase the capacitance. Such substances are liquid dielectrics on a synthetic basis, which are superior to petroleum oils.
Chlorinated hydrocarbons are formed from hydrocarbons by replacing hydrogen atoms with chlorine atoms. Polar biphenyl products, which contain C12 H10-nC Ln, are very popular.
Their advantage is resistance to combustion. One of the disadvantages is their toxicity. The viscosity of chlorinated biphenyls is high, so they have to be diluted with less viscous hydrocarbons.
Organosilicon liquids have low hygroscopicity and high temperature resistance. Their viscosity depends very little on temperature. Such liquids are expensive.
Organofluorine liquids have similar properties. Some fluid samples can operate at 2000 degrees for a long time. Such liquids in the form of octol consist of a mixture of isobutylene polymers obtained from petroleum cracking gas products and are low in cost.
Natural resins
Rosin is a resin that has increased fragility and is obtained from resin (pine resin). Rosin consists of organic acids, easily dissolves in petroleum oils when heated, as well as in other hydrocarbons, alcohol and turpentine.
The softening temperature of rosin is 50-700 degrees. In the open air, rosin oxidizes, softens faster, and dissolves less well. Rosin dissolved in petroleum oil is used to impregnate cables.
Vegetable oils
These oils are viscous liquids that are obtained from various plant seeds. The most important are drying oils, which can harden when heated. A thin layer of oil on the surface of the material, when dried, forms a hard, durable electrical insulating film.
The rate of oil drying increases with increasing temperature, lighting, and the use of catalysts—driers (compounds of cobalt, calcium, and lead).
Flaxseed oil has a golden yellow color. It is obtained from flax seeds. The pour point of linseed oil is -200 degrees.
Tung oil is made from the seeds of the tung tree. This tree grows in the Far East, as well as in the Caucasus. This oil is non-toxic, but not food grade. Tung oil hardens at a temperature of 0-50 degrees. Such oils are used in electrical engineering for the production of varnishes, varnished fabrics, wood impregnation, and also as liquid dielectrics.
Castor oil is used to impregnate capacitors with paper dielectric. This oil is obtained from castor bean seeds. It hardens at a temperature of -10 -180 degrees. Castor oil is easily soluble in ethyl alcohol, but insoluble in gasoline.
Ions
Their thermal motion consists of them oscillating somewhere around an equilibrium position. However, the interesting thing is that some of them are still able to break free and overcome what is holding them back. Such ions can be conventionally called free. They move to places where their potential energy will be very low. If we are talking about electrical dielectrics (and we are still talking about them), then such places in a dense crystal lattice for them are nodes.
So, according to Walter Schottky’s theory, this can only happen when a certain number of lattice sites are already occupied by ions. In physics, such nodes are often called “holes.” Then thermal motion will be reduced to a random jumping of ions from one site to another.
Dielectric once and for all?
When we call this or that substance a dielectric, we must understand that this name is rather arbitrary, because under a certain influence on the substance it can already lose the properties of a dielectric. Why is this happening?
The fact is that electric current affects the substance only for a very short period of time, which is why the field in it also does not appear for long. Therefore, even substances with very low resistivity can also be considered a dielectric under certain conditions.
A good example would be distilled water. But if the voltage affects the substance for a very long time, then it can already be safely called a conductor. This is such magic.
Customizable
Tunable Dielectrics
insulators whose ability to store electrical charge changes when voltage is applied.[12][13]
In general, strontium titanate (SrTiO 3) is used for devices operating at low temperatures, and barium strontium titanate (Ba 1-xSr xTiO 3) is used as a substitute for room temperature devices. Other potential materials include microwave dielectrics and carbon nanotube (CNT) composites.[12][14][15]
In 2013, multi-sheet layers of strontium titanate alternating with individual layers of strontium oxide produced a dielectric capable of operating at frequencies up to 125 GHz. The material is created through molecular beam epitaxy. These two crystals have a mismatch in crystal spacing, which creates stress in the strontium titanate layer, making it less stable and tunable.[12]
Systems such as Ba 1-xSr XTiO 3 have a paraelectric-ferroelectric transition just below ambient temperature, which provides high tunability. Such films suffer significant losses due to defects.
Amorphous dielectrics. What are they?
What is special about amorphous dielectrics? The main thing that distinguishes them from others is their rather loose structure, which means there are a lot of voids inside and a large space where the ions can be in a state of equilibrium. Moreover, during the transition from one equilibrium state to another, the energy consumed by the ion will always be different. In some transitions, the ion will not be completely freed from the forces restraining it, so it can be conditionally characterized as half-bound by these forces.
Such transitions will consume a very small amount of energy, and the ion can move only a very short distance during such transitions. As a result of thermal movement, such transitions inside amorphous bodies will occur much more often, because they require much less energy than others.
However, a small number of ions that contain large reserves of energy will still be able to overcome the forces that bind them and will move over relatively long distances.
If we draw an analogy with a crystal lattice, then these ions can be called free. As we have now found out, in general, this situation during the movement of ions in amorphous bodies is identical to solid ones, but with minor reservations.
Place it in a permanent field
Now let's move a little away from what substances can be dielectrics and what cannot be, especially since we have already understood this issue quite well.
Let's now try to answer this interesting question: what will happen if a dielectric is placed in a constant electric field? First, let's give a short answer, and then we will look at this issue in more detail. So, if you place a dielectric in an electric field, then the charges of the dielectric of which it consists will be under the influence of certain forces, which will be:
- displace bound charges (these are only electrons and ions)
- impose a field on the random movement of heat, which will order this movement (positive charges will go in one direction with the field, and negative charges in the opposite direction)
What will orderly movement provide?
When ordering dielectric charges, there are two possible scenarios:
- a new equilibrium state with a different distribution of charges, and the movement immediately stops when equilibrium is reached
- While the field is in effect, ordering can continue as long as there are still free electrons or free ions in it, which we talked about above
Recommendations
- ^ a b c
Dielectric.
Encyclopedia Britannica
: “A dielectric, an insulating material, or a very poor conductor of electric current. When dielectrics are placed in an electric field, virtually no current flows in them because, unlike metals, they do not have loosely bound or free electrons that can drift through the material." - Arthur R. von Hippel, in his seminal work Dielectric Materials and Applications
, stated: “
Dielectrics
... are not a narrow class of so-called insulators, but a wide range
of nonmetals
are considered from the point of view of their interaction with electric, magnetic or electromagnetic fields. Thus we are dealing with gases as well as liquids and solids, and with the storage of electrical and magnetic energy, as well as its dissipation" (p. 1) (Technology Press of MIT and John Wiley, NY, 1954) . - Thoms, E.; Sippel, P.; and others. (2017). "Dielectric studies of mixtures of ionic liquids". Sci.
Representative .
7
(1): 7463. arXiv:1703.05625. Bibcode:2017NatSR ... 7.7463T. doi:10.1038/s41598-017-07982-3. PMC 5547043. PMID 28785071. - Belkin, A.; Bezryadin, A.; Hendren, L.; Hoobler, A. (2017). "Recovery of aluminum oxide nanocapacitors after high and low voltage breakdown." Sci.
Representative .
7
(1): 932. Bibcode:2017NatSR ... 7..932B. Doi:10.1038/s41598-017-01007-9. PMC 5430567. PMID 28428625. - Daintith, J. (1994). Biographical Encyclopedia of Scientists
. CRC Press. item 943. ISBN 978-0-7503-0287-6. - James, Frank A.J.L., editor. The Correspondence of Michael Faraday, Volume 3, 1841–1848, "William Whewell Letter to Faraday 1798, p. 442." Archived from the original on 2016-12-23. Retrieved 2012-05-18. Institution of Electrical Engineers, London, UK, 1996 ISBN 0-86341-250-5
- Microwave Engineering - R. S. Rao (Prof.)
. Retrieved 2013-11-08. - Kao, Kwan Chi (2004). Dielectric phenomena in solids
. London: Elsevier Academic Press. pp. 92–93. ISBN 978-0-12-396561-5. - Debye, P. (1913), Ver. Deut. Phys. Gesell. 15, 777; republished in 1954 in a collection of articles by Peter J. W. Debye. Interscience, New York
- Chan, Y. et al (1997) Physical Ceramics
, John Wiley and Sons, New York - Kuhn, U.; Luthi, F. (1965). "Paraelectric heating and cooling by OH dipoles in alkali metal halides". Solid State Communications
.
3
(2): 31. Bibcode:1965SSCom ... 3 ... 31K. Doi:10.1016/0038-1098(65)90060-8. - ^ a b c
Lee, Che-Hui;
Orloff, Nathan D.; Birol, Turan; Zhu, Ye; Goyan, Veronica; Rokas, Edward; Heislmeier, Ryan; Vlachos, Eftyhia; Mundy, Julia A.; Kourkoutis, Lena F.; Nie, Yuefeng; Biegalski, Michael D.; Zhang, Jingshu; Bernhagen, Margitta; Benedek, Nicole A.; Kim, Yongsam; Brock, Joel D.; Uecker, Reinhard; Xi, X. X.; Gopalan, Venkatraman; Nuzhny, Dmitry; Kamba, Stanislav; Mueller, David A.; Takeuchi, Ichiro; Booth, James S.; Fenny, Craig J.; Schlom, Darrell G. (2013). “Self-correcting crystal could lead to the next generation of advanced communications.” Nature
.
502
(7472):532–6. Bibcode:2013Natura.502..532L. doi:10.1038/nature12582. PMID 24132232. S2CID 4457286. - Lee, CH.; Orlov, N. D.; Birol, T.; Zhu, Y.; Goian, V.; Rocas, E.; Haislmaier, R.; Vlahos, E.; Mundy, J. A.; Kourkoutis, L. F.; Nie, Y.; Biegalski, M.D.; Zhang, J.; Bernhagen, M.; Benedek, N. A.; Kim, Y.; Brock, J.D.; Uecker, R.; Xi, X. X.; Gopalan, V.; Nuzhny, D.; Kamba, S.; Mueller, D. A.; Takeuchi, I.; Booth, J. S.; Fennie, C.J.; Shlom, D. G. (2013). "Using Dimensionality and Defect Elimination to Design Tunable Microwave Dielectrics". Nature
.
502
(7472):532–536. Bibcode:2013Natura.502..532L. doi:10.1038/nature12582. HDL:2117/21213. PMID 24132232. S2CID 4457286. - Kong, L.B.; Li, S.; Zhang, T.S.; Zhai, J. W.; Boey, F.Y.C.; Ma, J. (November 30, 2010). "Electrically tunable dielectric materials and strategies for improving their performance." Progress in Materials Science
.
55
(8):840–893. Doi:10.1016/j.pmatsci.2010.04.004. - Giere, A.; Zheng, Y.; Maune, H.; Sazegar, M.; Paul, F.; Zhou, X.; Binder, J. R.; Muller, S.; Jacobi, R. (2008). "Tunable Dielectrics for Microwave Applications". 2008 17th IEEE International Symposium on Ferroelectric Applications
. paragraph 1. Doi:10.1109 / ISAF.2008.4693753. ISBN 978-1-4244-2744-4. S2CID 15835472. - Müssig, Hans-Joachim. Semiconductor capacitor with praseodymium oxide as dielectric
, US Patent 7,113,388 published 11/06/2003, issued 10/18/2004, assigned to IHP GmbH - Innovations for High Performance Microelectronics / Institute Fur Innovative Mikroelektronik - ^ a b
Cole, M. W.;
Geyer, R. G. (2004). "Novel Tunable Acceptor-Doped BST Thin Films for High-Performance Tunable Microwave Devices." Revista Mexicana de Fisica
.
50
(3): 232. Bibcode:2004RMxF … 50..232C. - Nair, K. M.; Guo, Ruyan; Bhalla, Amar S.; Hirano, S.-I.; Suvorov, D. (2012-04-11). Developments in Dielectric Materials and Electronic Devices: Proceedings of the 106th Annual Meeting of the American Ceramic Society, Indianapolis, Indiana, USA, 2004
. John Wiley and Sons. ISBN 9781118408193. - Nair, K. M.; Bhalla, Amar S.; Hirano, S.-I.; Suvorov, D.; Schwartz, Robert W.; Zhu, Wei (April 11, 2012). Ceramic materials and multilayer electronic devices
. John Wiley and Sons. ISBN 9781118406762. - Cole, M.W.; Hubbard, S.; Ngo, E.; Erwin, M.; Wood, M.; Geyer, R. G. (July 2002). "Structure-property relationships in pure and acceptor-doped Ba1-xSrxTiO3 thin films for tunable microwave devices." Journal of Applied Physics
.
92
(1):475–483. Bibcode:2002JAP…. 92..475C. doi:10.1063/1.1484231. ISSN 0021-8979. - Lyon, David (2013). "Dependence of the dielectric strength of nano-vacuum gaps on the gap size." IEEE Transactions on Dielectrics and Electrical Insulation
.
20
(4): 1467–1471. doi:10.1109/TDEI.2013.6571470. S2CID 709782.
Let's talk about polarization
The next important term that it’s time to learn about is the polarization of dielectrics. The fact is that the processes of displacement of dielectric charges occur at different speeds. As we said earlier, for bound charges the displacement time is much shorter, but other processes proceed very slowly.
When the dielectric charges are displaced, another field is formed. It just makes the main (external) field weaker. It is precisely the phenomenon of the formation of a new field that is called polarization of the dielectric. Now let's delve into this process, because there are a lot of interesting details.
First, let's understand why a new field appears at an offset. Everything is simple here, because now from a disordered state the dielectric becomes more ordered - the negative charges are now located to the left of their positive charges. This is precisely what creates a new field.
Dielectric permeability
But how can we measure how much the internal field weakens the external one? Well, everything is very simple here. This measure is called electrical permeability or dielectric permeability (you've probably already heard this term). They usually say that the permeability of a dielectric is constant, but in fact, due to the fact that polarization takes quite a long time, we will say that this value depends on the time of action of the external field.
How does temperature affect the permeability of a dielectric?
But is it only time that affects electrical conductivity? It turns out that not only. It turns out that if you increase the temperature, then at the same time the intensity of thermal movement also increases, and this, as you understand, directly affects the permeability of the dielectric. Why? It's simple: the transition to a stable state becomes more difficult, and therefore the dielectric constant becomes less and less with increasing temperature.
Electrical porcelain
It is the most common ceramic electrical insulating material. The composition of porcelain includes: kaolin - white clay, fire clay, quartz and feldspar. The production of porcelain products consists of the following operations: grinding the components of porcelain and mixing them with water into a homogeneous mass. By pressing, grinding, casting into plaster molds or extruding from this mass, products of the desired configuration are obtained. To remove excess water, the products are dried, then they are covered with a glassy mass - glaze, which reduces the hygroscopicity of porcelain, gives a certain color to the products and creates an even, smooth surface during firing. After glazing, the product is dried again and fired in ovens at a temperature of 1320 - 1450 ° C. Porcelain is characterized by high heat resistance, resistance to electric arcs and very low water absorption. Linear (suspension and pin) insulators, stationary (support and bushing) insulators, hardware insulators, installation porcelain products (rollers, parts of fuses, cartridges, plugs, etc.) are made from porcelain. Electric strength of porcelain 6 – 10 kV/mm; ε = 5 – 6.5. In addition to porcelain, another ceramic material is used - steatite, made on the basis of the mineral - talc. Soapite, compared to porcelain, has higher electrical insulating and physical-mechanical properties.
| Materials on electrical conductivity properties |
Dielectric breakdown
Remember, in this article we already said that each dielectric has its own limit and that a substance cannot be unambiguously called a dielectric and must be considered in dynamics. So, let's return to this topic and go a little deeper into it. Do you know what happens during polarization?
The fact is that with this phenomenon, a state begins, called stationary or quasi-stationary, if the influence of voltage from the outside is variable. This state differs from the usual one in that the polarization values can remain at the same level for a very long time. Together with them, electrical conductivity also stabilizes.
If you immediately begin to increase the tension in such a field, then it will be possible to very accurately determine the limit at which this very stability will be sharply violated. The current and electrical conductivity will immediately increase, and this is a direct path from the dielectric to the conductors. Indeed, after this the substance can no longer be characterized as a dielectric. This process of transition of a dielectric into conductors is called dielectric breakdown.
Now that we have understood what breakdown is, let us now understand how we can easily determine at what point dielectric breakdown occurs. As we can understand, the breakdown time threshold may depend on temperature, the state of aggregation of the substance and many other factors; something else is important here. Let's look at the main cases of breakdown, there are only two of them, so don't be alarmed:
- thermal phenomena in which an increase in electrical conductivity is caused by the fact that the dielectric heats up very quickly, due to which the thermal state can no longer be stationary
- electrical phenomena that occur due to an increase in the number of free electrons and ions. This also happens in two cases. Either the appearance of free charges is due to their being knocked down by other moving charges, or due to being knocked down by a field.
Electrical insulating materials and areas of their application
The main areas of application of electrical insulating materials include various industrial branches, radio engineering, instrument making and installation of electrical networks. Dielectrics are the main elements on which the safety and stability of any electrical appliance depends. The quality and functionality of insulation is influenced by various parameters.
Thus, the main reason for using electrical insulation is to comply with safety regulations. In accordance with them, it is strictly prohibited to operate equipment with partially or completely missing insulation or damaged shell, since even small currents can harm the human body.
Field in a dielectric
As we already understood, the field in the dielectric is directed exactly opposite the external electric field. But this knowledge is not enough for us to have a good understanding of dielectrics.
So let's delve a little deeper into this topic. Let us recall that the polarization of a dielectric is when the charges are redirected so that the minuses face one way and the pluses face the other. So, let's look at the types of polarization.
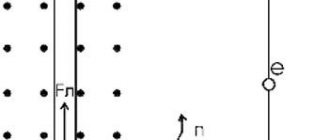
Deformation (or electronic)
This type of polarization interests us most. It is worth noting that such polarization is typical for substances consisting of non-polar molecules, that is, which do not have dipole moments. What's happening? It's simple - the main thing you need to understand is that the electron shells shift. In this case, positively charged atomic nuclei are displaced towards the external field, and negatively charged electron shells are shifted against the field.
Dipole (or orientation)
This is one of the most common types of polarization. However, here everything is exactly the opposite. Here the orientation of the dipoles is already changing. It’s still simple here - when the field from outside does not affect the substance, the order of the dipoles is absolutely chaotic, but when the external field begins to influence the substance, then absolutely all dipoles turn with their positive side towards the field that influences it. As we have already discussed above, the stability of the position of the dipoles is determined by the field strength and temperature of the substance.
Ionic
Yes, we haven’t forgotten this type of polarization either. Here we are talking about the displacement of the positive lattice of ions. They will be located along the field, and negative ones - against.
So why did we say at the very beginning that we would be most interested in the first type of polarization if we consider positive charges? It's simple. Positive charges play some role only under such an influence of an external field on a substance. Therefore, you can assume that you already know everything you need to know about them.
Corona discharge.
Also on topic:
ELECTRIC ENERGY
One of the most famous and widespread insulators is air at atmospheric pressure and normal temperature. For low voltages, the electrical resistivity of such air is approx. 1018 OhmHsm. When the electric field strength across a uniform air gap reaches 30 kV/cm, the conductivity increases as photoionization of the air begins and eventually a spark jumps between the electrodes. If the electrode geometry is heterogeneous, as in the case of a tip and a plane or a power line wire above the surface of the earth, a glowing region of ionized air called a corona discharge appears around the tip or wire when the electric field strength is high enough. The corona current increases with increasing voltage and eventually a spark or arc occurs depending on the power of the source and the resistance of the external circuit.
Flat dielectric
For some reason, many people sometimes call the dielectric inside a flat-plate capacitor. Perhaps it’s just more convenient to call it that way. In fact, a flat capacitor is a very interesting device, so let's talk about it and its dielectric (flat dielectric for that matter).
Since we are talking about a capacitor, let's immediately learn how to determine its capacitance (or the capacitance of a dielectric). To do this, let's use this wonderful formula:
Let's understand what each of the letters means here. S is obviously the area of the plates of a given parallel-plate capacitor. The letter d denotes the distance between the plates, and the other two variables are the dielectric constant of the dielectric (planar dielectric) and the electrical constant (in case some of you have forgotten, 8.854 pF/m)
It’s strange, but now flat capacitors are very rare. This may be due to film technology, which is so microscopic that it is quite difficult and expensive to make.
Why can’t a flat capacitor and a dielectric live without each other?
The answer to this question is not that complicated. The thing is that the most important and basic element in a flat capacitor—its capacitance—depends on the dielectric. Let's talk about how it works. As we know, an amorphous substance consists of dipoles, which, in turn, are fixed in place and randomly oriented.
When an external field acts on this most amorphous substance, the dipoles unfold along the lines of force of this external field. At the same time, the field weakens, and the charge gradually accumulates until the field ceases to act. And so it goes on cycle after cycle. That is why a flat capacitor with a dielectric can only be considered together.
Applications
Capacitors
Main article: Capacitor
The separation of charges in a parallel plate capacitor produces an internal electric field. The dielectric (orange) reduces the field and increases the capacitance.
Commercially produced capacitors typically use a solid dielectric material with a high dielectric constant as an intermediate medium between the accumulated positive and negative charges. This material is often referred to in a technical context as a capacitor dielectric
.[16]
The most obvious advantage of using such a dielectric material is that it prevents direct electrical contact between the conductive plates on which the charges are stored. More importantly, however, high dielectric constant allows more charge to be stored at a given voltage. This can be verified by considering the case of a linear dielectric with permittivity ε
and the thickness
d
between two conducting plates with uniform charge density
σε
.
In this case, the charge density is given by σ ε = ε V d {displaystyle sigma _ {varepsilon} = varepsilon {frac {V} {d}}}
and the capacitance per unit area at
c = σ ε V = ε d {displaystyle c = {frac {sigma _ {varepsilon}} {V}} = {frac {varepsilon} {d}}}
From this it is easy to see that the larger ε
results in more stored charge and therefore more capacity.
The dielectric materials used for capacitors are also selected to be resistant to ionization. This allows the capacitor to operate at higher voltages before the insulating dielectric ionizes and allows unwanted current to pass through.
Dielectric resonator
Main article: Dielectric resonator
A generator with a dielectric resonator
(DRO) is an electronic component that exhibits a polarization response resonance for a narrow range of frequencies, typically in the microwave range. It consists of a ceramic “puck”, which has a high dielectric constant and low dissipation coefficient. Such resonators are often used to set the frequency in the generator circuit. An unshielded dielectric resonator can be used as a dielectric resonator antenna (DRA).
BST Thin Films
From 2002 to 2004 The Army Research Laboratory (ARL) conducted research on thin film technology. Barium strontium titanate (BST), a ferroelectric thin film, has been explored for the fabrication of radio frequency and microwave components such as voltage controlled oscillators, tunable filters, and phase shifters.[17]
The research was part of an effort to provide the Army with highly tunable, microwave-compatible materials for broadband electric field tunable devices that operate stably at extreme temperatures.[18] This work improved the ability to tune bulk barium strontium titanate, which is a thin-film activator for electronic components.[19]
In a 2004 research paper, ARL researchers examined how small concentrations of acceptor impurities can dramatically change the properties of ferroelectric materials such as BST.[20]
The researchers "doped" BST thin films with magnesium, analyzing "the structure, microstructure, surface morphology, and quality of the film/substrate composition." Magnesium-doped BST films have shown "improved dielectric properties, low leakage current, and good tunability", allowing their use in tunable microwave devices.[17]
How not to confuse conductors and dielectrics
Before this, we looked at dielectrics in great detail, learned how they work, how they are structured inside. Now let's find out how they are used in real life and how not to confuse them with conductors.
Where are dielectrics used?
Dielectrics are used in many areas of life, namely in those where electric current is needed.
They are especially actively used in agriculture, industry and instrument making.
Solid dielectrics
Dielectrics are different. For example, solid dielectrics can ensure the safety of devices that run on electricity. They are good current insulators, which means they greatly influence the durability of these devices. One example is dielectric gloves.
Liquid dielectrics
But liquid dielectrics are needed for a slightly different purpose. They are used in capacitors, cables, air-circulating cooling systems and many other devices.
Heat resistance classes of electrical insulating materials
The heat resistance class of dielectrics is indicated by a letter of the Latin alphabet. We list the main ones:
- Y – maximum temperature 90 degrees. Celsius. This category includes various fiber products made from cotton, natural fabrics and cellulose. They are not impregnated or supplemented with liquid electrical insulators.
- A – 105 deg. Celsius. All materials listed above and synthetic silk are impregnated with liquid dielectrics (immersed in them).
- E – 120 degrees. Celsius. Synthetic products including fibres, films and compounds.
- B – 130 degrees. Celsius. Mica dielectrics, asbestos and fiberglass, coupled with an organic binder and impregnation.
- F – 155 degrees. Celsius. Mica materials, the connecting link of which is synthetic components.
- H – 180 deg. Celsius. Mica dielectrics with organosilicon compounds acting as a binder.
- C – more than 180 degrees. Celsius. All products listed above that do not use a binder or use inorganic adhesives.
The choice of electrical insulating materials depends not only on the capacity of the equipment, but also on its operating conditions. For example, for high-voltage power lines, dielectrics with increased frost resistance and protection from ultraviolet rays must be used.
Therefore, the information above should be used for informational purposes only and the final decision should be made by a professional, qualified professional.