Content
In the last lesson we already mentioned conductors and dielectrics. We defined them as substances in which free electrons are present or absent. They are the ones who carry out the transfer of electrical charge. Conductors have them, but dielectrics do not.
And yet, the main feature that we will consider is the ability to conduct current or transmit electric charge. Based on this ability, substances are divided into three main classes: conductors, semiconductors and dielectrics . In this lesson we will define each class of substances, consider the nature of semiconductors, which we have not encountered before.
Conclusions website
- In a conductor, free electrons, influenced by the forces of the electric field, move throughout the entire volume.
- Unlike a conductor, there are no free charges in a dielectric (insulator). Insulators consist of neutral molecules or atoms. The charges in a neutral atom are strongly bound to each other and cannot move under the influence of an electric field throughout the entire volume of the dielectric.
Electronic devices use a variety of materials. The main elements used for these devices are conductor and semiconductor products. To use them more effectively, you need to know exactly how conductors differ from semiconductors. The properties of each element, used in combination, make it possible to create devices with unique qualities and characteristics.
Conductors
Let's start with a definition.
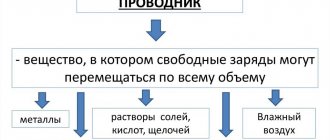
A conductor is a body through which electric charges can pass from a charged body to an uncharged one.
Examples of conductors:
- metals
- the soil
- water with salts, acids or alkalis dissolved in it
- graphite
Figure 1. Metals are the best conductors of electricity.
The best conductors are metals (Figure 1). Silver, copper and aluminum have the highest conductivity.
Our bodies also conduct electricity. We are very unique guides. This can be easily checked by touching any charged body, for example, the petals of an electroscope. The charge will transfer to us, and then go through the floor into the ground.
In all these substances and in our body there are free electrons, which carry charge.
World of Science
All substances are made up of atoms and molecules with positively charged nuclei and negatively charged electrons. Atoms and molecules are electrically neutral because the charge on the nucleus is equal to the total charge
electrons surrounding the nucleus. In the presence of external factors (increased temperature, electric field, etc.), an atom or molecule loses an electron. This atom turns into a positive ion, and an electron detached from the atom can join another atom, turning it into a negative ion, and remain free. The process of ion formation is called ionization. The number of free electrons or ions per unit volume of a substance is called the concentration of charged particles. Thus, in a substance that is placed in an electric field, under the influence of field forces, a process of movement of free electrons or ions in the direction of the field forces occurs, called electric current.
The property of a substance to conduct current under the influence of an electric field is called the electrical conductivity of the substance, which depends on the concentration of free electrically charged particles. The greater the concentration of charged particles, the greater the electrical conductivity of the substance. All substances, depending on electrical conductivity, are divided into:
1 Conductor. They have very high electrical conductivity. Conductors are divided into two groups. The first group of conductors includes metals (copper, aluminum, silver, etc.) and their alloys, in which only electrons can move. That is, in metals, electrons are very weakly bound to the nuclei of atoms and are easily separated from them. In metals, the phenomenon of electric current is associated with the movement of free electrons, which have very high mobility and are in a state of thermal motion. This electrical conductivity is called electronic conductivity. Conductors are used for the manufacture of wires, power lines, windings of electrical machines, etc. The second group of conductors includes aqueous solutions of salts, acids, etc., which are called electrolytes. Under the influence of the solution, the molecules of the substance break up into positive and negative ions, which begin to move under the influence of the electric field. When a current passes, electrolyte ions will begin to precipitate on the electrodes immersed in the electrolyte. The process of separating a substance from electrolytes by electric current is called electrolysis. It is used to extract non-ferrous metals from solutions of their compounds (copper, aluminum), as well as to coat metals with a protective layer of another metal (for example, chrome plating).
2 Dielectrics (or electrical insulating substances). Substances with very low electrical conductivity (gases, rubber substances, mineral oils, etc.). In these substances, electrons are very tightly bound to the atomic nuclei and are rarely separated from the nuclei under the influence of an electric field. Those. Dielectrics do not conduct electricity. This property is used in the production of electrical protective equipment: dielectric gloves, shoes, mats, insulating stands, pads, caps, insulators on electrical equipment, etc.
Dielectrics can be: solid, gaseous, liquid.
3 Semiconductor (germanium, selenium, silicon). These are substances that, in addition to electronic conductivity, have “hole” conductivity, which largely depends on the presence of external factors: light, temperature, electric or magnetic field. These substances have a covalent bond (a chemical bond between two electrons of neighboring atoms in the same orbit). The covalent bond is very weak. In the presence of an external factor, it is destroyed and free electrons appear (electronic conductivity). At the moment of formation of a free electron in a covalent bond, a free city appears - an “electron hole” (equivalent to a proton), which attracts an electron from a neighboring covalent bond. But then a new “hole” is formed, which again attracts an electron from a neighboring covalent bond, and so on. Those. under the influence of an electric field, “holes” move in the direction of the field (towards the electrons) - the movement of protons. Thus, with electronic conduction, the electron travels the entire path, and with “hole” conduction, electrons are alternately replaced along bonds, each electron travels a fraction of the path. When bonds in semiconductors are broken, the same number of electrons and “holes” appear simultaneously. That is, conductivity consists of electron and “hole” and is called the intrinsic conductivity of a semiconductor. The properties of semiconductors can be changed if impurities of other substances are added to them. Thereby increasing one or another conductivity. This is used in industrial electronics: diodes, transistors, thyristors. They are used as amplifiers, rectifiers, electronic generators, stabilizers and the like. Their advantages: low energy loss, cost, size and weight, ease of operation, long service life. Disadvantage: dependence of conductivity on temperature.
Non-conductors
Let's give a definition.
A dielectric/non-conductor is a body through which electric charges cannot pass from a charged body to an uncharged body.
Examples of dielectrics:
- ebonite
- amber
- rubber
- porcelain
- plastic
- silk
- oil
- nylon
- air and gases
- glass
- dry wood
Figure 2. Dry wood is an excellent dielectric.
All these substances have in common the absence of free electrons. They are also used for the manufacture of isolates or insulation .
Repetition
In this lesson, we will continue to get acquainted with the topic “Electrical Phenomena”, and consider issues related to the conductivity and non-conductivity of electric charge materials, and also get acquainted with the first simple instruments for measuring and recording charges - an electrometer and an electroscope.
In the previous lesson, we found out that electrical phenomena exist, that they can be observed, and that they are associated with the interaction of different charges. We also found out that these interactions are determined by the action of force, and, accordingly, the magnitude of the interaction is determined by the magnitude of the electric charge. We also learned that like charges repel, and unlike charges, on the contrary, attract.
Now we have to get acquainted with how these electric charges can move and pass from one body to another.
Semiconductors
These bodies are intermediate in their ability to transfer electrical charges between conductors and dielectrics.
A semiconductor is a body that does not conduct electricity at low temperatures, but begins to conduct electricity at higher temperatures.
What does this mean? The fact is that at low temperatures semiconductors are dielectrics. They are not capable of transmitting any charge.
Let's raise the temperature. The atoms of a substance begin to vibrate more strongly around their equilibrium positions. These vibrations reach such a force that the electrons located on the outer shells of the atoms (valence electrons) become free. This is how a semiconductor becomes a conductor.
What characteristic feature do semiconductors have? As the temperature increases, their conductivity increases . Interestingly, for metals, on the contrary, it will decrease.
Note that this temperature is not always very high. For example, for silicon and germanium it is about $20 \degree C$.
Examples of semiconductors:
- metal oxides and sulfates
- germanium
- silicon
- some organic matter
Due to their properties, semiconductors are widely used in technology. They are often used as a kind of thermometer. For example, they are used as temperature-dependent resistors. This allows you to control the flow of current at certain temperatures. When it reaches a critical point, some part of the circuit stops conducting current or, conversely, begins. We will talk in more detail about the electrical circuit and its components in the following lessons.
Semiconductors begin to conduct electricity under other influences on them:
- exposure to light
- transmission of a stream of fast particles
- introduction of impurities
Figure 3. When exposed to light, semiconductors begin to conduct electricity.
Photoconductivity is the phenomenon of increased conductivity of a substance when exposed to light.
This phenomenon allows semiconductors to be used in remote control and alarm systems. We can say that the scope of semiconductors in technology is itself very wide. They are an integral part of microcircuits in televisions, computers, radios, and are used to create transistors, diodes, etc.
What is a semiconductor
A semiconductor conducts electric current, but not like metals, but subject to certain conditions - imparting energy to the substance in the required quantities. This is due to the fact that there are too few free charge carriers (holes and electrons) or none at all, but if you apply a certain amount of energy, they will appear. Energy can be of various forms - electrical, thermal. Also, free holes and electrons in a semiconductor can appear under the influence of radiation, for example in the UV spectrum.
Where are semiconductors used? Transistors, thyristors, diodes, microcircuits, LEDs, etc. are made from them. Such materials include silicon, germanium, mixtures of different materials, such as gallium arsenide, selenium, and arsenic.
To understand why a semiconductor conducts electricity but not like metals, we need to consider these materials from the point of view of band theory.
Conductivity and electrification
Let us note an important point. You should never confuse electrification and conductivity.
Bodies that are not conductors may well have the ability to become electrified.
Electrification occurs through direct contact of bodies. Conduction occurs inside the body.
During electrification, one body loses electrons and the other gains. Conduction or electric current (more on that in the next lesson) describes the orderly movement of particles within a body.
Properties of conductors and semiconductors
Many substances are capable of conducting electric current. They can be in solid, liquid or gaseous state. The main conductors used in electrical engineering are various types of metals or their alloys. They are distinguished by high conductivity qualities and electrical resistance characteristic of each material.
In electrical engineering, metals are used as conductors, structural and contact materials, as well as for soldering all types of conductors together. The main property of conductors is the presence of free electrons in them, which ensure the passage of electric current.
The category of semiconductors includes substances that occupy an intermediate position between. These boundaries are quite arbitrary, since under the influence of various factors, semiconductors can have the properties of both conductors and insulators. For example, under the influence of low temperatures, they become dielectrics, and when the temperature rises, free charge carriers begin to appear in them. This is due to the fact that as the temperature rises, the vibrations of the crystal lattice also increase, breaking certain valence bonds and forming free electrons that conduct electric current.
Exercises
Exercise No. 1
Why does a charged electroscope discharge when its ball is touched by hand?
Our body is a conductor of electricity . When we touch the ball of a charged electroscope, the charge (free electrons) goes into our body. When we come into contact with the floor and the ground, the charge will go there. This happens if the electroscope is negatively charged.
If the electroscope is positively charged, then by touching it, we neutralize the charge by giving it a certain number of electrons. After all, being a conductor, our body has a large number of free electrons.
Exercise No. 2
Why is the rod of an electroscope made of metal?
Metals are good conductors. The metal rod can transfer charge from the ball to the petals.
If you make a rod from a dielectric, then the charge will not be transferred, and the electroscope will not work.
Exercise #3
A positively charged body is brought to the ball of an uncharged electroscope without touching it.
What charge will appear on the leaves of the electroscope? Note that the body is not touching the electroscope. As it approaches, a negative charge is formed on the ball, and a positive charge on the petals.
The electric field of a positively charged body will act on the electroscope, free electrons will begin to move. The attractive forces between unlike charges will force them to gather on the ball. In another part of the electroscope (on the petals), a lack of electrons is formed and a positive charge is formed.