Conductors
Conductors are characterized by good electrical conductivity, that is, a large number of free electrically charged particles (electrons or ions) that can move under the influence of field forces along the conductor.
Conductors of the first kind
There are two kinds of conductors. Conductors of the first kind, in which only electrons can move, are metals. In metallic conductors, electrons located in the outer orbits of atoms are relatively weakly coupled with their nuclei, which is why some of the electrons separated from their nuclei move between the atoms, moving from the sphere of action of one nucleus to the sphere of action of another and filling the space between them like a gas. These electrons are usually called free electrons or conduction electrons. Free electrons are in a state of random (thermal) motion, in contrast to the positively charged metal ions that make up the core of the conductor, which have very low mobility and make only small oscillations around their average position.
Conductors of the second kind
In conductors of the second type, called electrolytes (aqueous solutions of acids, salts, alkalis and bases), under the influence of a solvent, the molecules of the substance break up into negative and positive ions, which, like electrons in metal conductors, can move throughout the entire volume of the conductor.
Conductors and dielectrics. Types of conductors
The electron has the least negative charge.
For reference: the electron charge is e0 = -1.6021766208*10-19 Coulomb
An electron (if it is weakly bound to the nucleus of an atom) can leave the atom, move into the interatomic space, enter the boundaries of another atom, etc. This phenomenon is most characteristic of metals. Metals always contain a huge number of electrons moving randomly in the interatomic space, called free (Figure 1).
Figure 1. Chaotic movement of electrons in a metal.
If we somehow order the movement of free electrons, that is, force them to move in one specific direction, then we will obtain an electric current in the metal (Figure 2).
Figure 2. The occurrence of current in a conductor.
Definition: Bodies with free electrons are called conductors of the first kind.
In conductors of the first type, the passage of electric current does not cause chemical changes in their substance. Conductors of the first kind include metals and their alloys. Conductors of the first type have found the widest application in electrical engineering and radio engineering. Wires, tires, capacitor plates, filaments of incandescent lamps and other conductive parts - all this is made from conductors of the first kind.
Definition: Conductors of the second type include solutions of acids, alkalis and salts.
Conductors of the second type are often called electrolytes. In the electrolyte, a continuous process of formation of negatively and positively charged molecules (ions) occurs. Electric current in an electrolyte represents the ordered movement of these ions (and not electrons, as was the case in conductors of the first kind).
Figure 3. Current in conductors of the second type (electrolytes).
Finally, there is a large group of substances that have neither free electrons nor ions. In such substances, under normal conditions, electric current cannot pass, and they are called dielectrics (porcelain, rubber, mica, glass, etc.).
Definition: Dielectrics are substances that do not have free electrons.
Dielectrics are widely used in modern electrical engineering as insulators (porcelain insulators on power lines, rubber coatings on wires, mica spacers, etc.).
DID YOU LIKE THE ARTICLE? SHARE WITH YOUR FRIENDS ON SOCIAL NETWORKS!
Related materials:
- The structure of matter or what matter is made of
- Electronic structure of the atom
- Electric current in a conductor
- Resistance to electric current.
Comments
Zalim 05/02/2019 05:15 Helpful
Quote
Update list of comments
Add a comment
Dielectrics
Substances in which the number of free electrons is negligible are called non-conductors (dielectrics or insulators). These include gases, some liquids (mineral oils, varnishes) and almost all solids, with the exception of metals and coal.
The best non-conductor of electric current is vacuum. Gases, including air, are also good insulators.
Conductors of the first and second kind
School textbook:
“In conductors of the first kind, electrons move between atoms and cations of the metal, which only oscillate around the equilibrium position. In conductors of the second type - electrolytes - the charge during the flow of electric current is carried by ions - particles of the substance itself. It is not the current (but, in the usual sense, the flow of electrons) that flows in the substance, but the substance itself moves, flows, pushed by the electric field. Moreover, particles of this substance - cations and anions - rush in different directions. Ions that have a positive charge move towards the negatively charged cathode (which is why they are called “cathode ions” - cations), and negative ions move towards the positively charged anode (anodic ions - anions).”
— — — — — — — —
Russian theory:
First, some general notes:
Metals do not have cations. Metal atoms do not “oscillate around an equilibrium position.” Charges don't exist. The “substance itself” is not pushed by the electric field. There is no electric field itself. Non-existent cations and anions do not “rush in different directions” under the influence of electrical forces. There is neither electrical attraction nor electrical repulsion, that is, there are no electrical forces.
Conductivity of metals. Metal atoms have contour grooves. When atoms stick together in the same grooves, they form continuous chains. Electrons slide along them. They shift down the slope of the electron pressure.
Electrons slide along the trough without energy loss, since they do not approach or move away from the trough. Difficulties arise when overcoming the joints of the gutters. Electrons can be pushed at the junctions by external pressure (electrical voltage) or rolling waves of radiation.
Conductivity of electrolytes. Pure mechanics also operates in an electrolyte. Features of the electrolyte are that:
* electrolyte molecules do not have contour grooves and therefore do not form conductive chains;
* an electrolyte molecule consists of an acidic component to which a metal atom or a hydrogen molecule is attached (molecular hydrogen is also a metal);
* the metal atom is almost always attached to the acidic component asymmetrically; therefore, when separated, its reactive force turns the acidic component in one direction, and under the influence of random shocks of neighboring molecules it can turn in the opposite direction; thus, the electrolyte molecule performs rotational movements;
* electrolyte – liquid (not solid or gaseous); Only in this state do the electrolyte molecules maintain contact with each other and can perform rotational movements.
The movement of electrons begins at the cathode.
Under the pressure of electron pressure, they move in groups to those electrolyte molecules that are adjacent to the cathode with their metal atoms (more precisely, those particles in the composition of the electrolyte molecules that have a convex groove). Acting like a wedge, the electrons tear these atoms away. The reactive force turns the remaining part of the molecule so that its opened trough turns in the opposite direction from the cathode. Electrons from the opened trough jump to the next molecule and again, like a wedge, tear off a metal atom from it. Under the action of an abstraction impulse, it approaches the acidic residue of the previous molecule and combines with it. Purely by chance, in the turmoil of turning particles, some of the reduced molecules turn out to be turned back towards the cathode. The process is repeated.
Thus, electrons move (jump) from molecule to molecule of the electrolyte and are directed from the cathode towards the anode. In this case, metal atoms make transitions from one acidic component to another in the opposite direction.
In this process there is no electron transfer between the electrolyte particles; acidic components remain in place; they just turn back and forth.
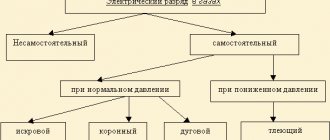
Conductors and dielectrics in an electric field
However, under certain conditions, for example in a strong electric field, dielectric molecules split into ions, and a substance that was an insulator in the absence of an electric field or in a weak field becomes a conductor. The electric field strength at which ionization of dielectric molecules begins is called the breakdown strength (electric strength) of the dielectric. The amount of electric field strength that is allowed in a dielectric when used in an electrical installation is called the permissible strength. The permissible voltage is usually several times less than the breakdown voltage.
The electrical properties of gases are strongly influenced by pressure and temperature.
As an example, we give the values of breakdown voltage in sq. cm for some dielectrics: air - 30, mineral oil (transformer) - 50-150, electrical cardboard - 100, porcelain - 80-150, mica - 800-2000.
Conductors and dielectrics grade 8 video:
Chemicals-el.ru
Conductors
– substances that conduct electric current due to the presence of a large number of charges in them that can move freely (unlike insulators). They are of I (first) and II (second) kind. The electrical conductivity of type I conductors is not accompanied by chemical processes; it is caused by electrons. Type I conductors include: pure metals, i.e. metals without impurities, alloys, some salts, oxides and a number of organic substances. On electrodes made of type I conductors, the process of transfer of a metal cation into a solution or from a solution to the metal surface occurs. Type II conductors include electrolytes. The passage of current in them is associated with chemical processes and is caused by the movement of positive and negative ions.
Electrodes of the first kind.
In the case of metal electrodes of the first kind, such ions will be metal cations, and in the case of metalloid electrodes of the first kind, metalloid anions. Silver electrode of the first kind Ag+/Ag. It is answered by the reaction Ag+ + e- = Ag and the electrode potential
E Ag+ /Ag = Ag+ / Ag+b 0log a Ag+.
After substituting the numerical values of E 0 and b 0 at 25 oC:
An example of metalloid electrodes of the first kind is the selenium electrode Se2–/Se, Se + 2e– = Se2; at 25 oC E Se2–/Se0 = –0.92 – 0.03log a Se2–.
Electrodes of the second kind
- half-cells consisting of a metal coated with a layer of a sparingly soluble compound (salt, oxide or hydroxide) and immersed in a solution containing the same anion as the sparingly soluble compound of the electrode metal. Schematically, an electrode of the second kind can be represented as follows: AZ– /MA, M, and the reaction occurring in it is MA + ze = M + AZ–. Hence the equation for the electrode potential will be:
Calomel electrodes
- This is mercury coated with calomel paste and mercury in contact with a KCl solution.
Cl– / Hg2Cl2, Hg.
The electrode reaction is reduced to the reduction of calomel to metallic mercury and chlorine anion:
The potential of the calomel electrode is reversible with respect to chlorine ions and is determined by their activity:
At 25 °C, the potential of the calomel electrode is found using the equation:
Mercury sulfate electrodes
SO42–/Hg2SO4, Hg are similar to calomel with the only difference that the mercury here is covered with a layer of paste of Hg and mercuric sulfate, and H2SO4 is used as a solution. The potential of a mercury sulfate electrode at 25 oC is expressed by the equation:
Silver chloride electrode
is a Cl–/AgCl, Ag system, and its potential corresponds to the equation:
E Cl– /AgCl, Ag = E 0Cl–/AgCl, Ag –b lg a Cl–
or at 25 oC:
E Cl–/AgCl, Ag = 0.2224 – 0.0592 lg a Cl–.
see also
Quantitative analysis...
Synthesis of 1-hydroxy-3-adamantanecarboxylic acid nitrate The purpose of this course work is to synthesize 1-hydroxy-3-adamantane acid nitrate and study its properties. As with most carbon-chain and heterochain polymers, the starting materials are...
Radium, Ra French scientists Pierre and Marie Curie discovered that the waste remaining after the separation of uranium from uranium ore (uranium tar, mined in the city of Joachimsthal, Czech Republic) is more radioactive than pure ...
What substances are classified as conductors of the second kind?
To the conductors
II
are
electrolytes. The passage of current in them is associated with chemical processes and is caused by the movement of positive and negative ions.
Interesting materials:
How to clear memory on Nokia 5? How to clear RAM memory? How to clean a Huawei tablet? How to clean a Huawei tablet? How to clean a Samsung tablet? How to clean plastic in a car? How to clean plastic in a car interior? How to clean Play Market? How to clear your Instagram subscriptions? How to clear subscriptions on Insta?