What are conductors and dielectrics
Conductors are substances with free electrical charges that can move directionally under the influence of an external electric field. They have these features:
- metals and their melts;
- natural carbon (coal, graphite);
- electrolytes - solutions of salts, acids and alkalis;
- ionized gas (plasma).
The main property of materials: free charges - electrons in solid conductors and ions in solutions and melts, moving throughout the entire volume of the conductor, conduct an electric current. Under the influence of electrical voltage applied to the conductor, a conduction current is created. Resistivity and electrical conductivity are the main indicators of a material.
The properties of dielectric materials are opposite to conductors of electricity. Dielectrics (insulators) - consist of neutral atoms and molecules. They do not have the ability to move charged particles under the influence of an electric field. Dielectrics in an electric field accumulate uncompensated charges on the surface. They form an electric field directed into the insulator, polarization of the dielectric occurs.
As a result of polarization, charges on the surface of the dielectric tend to reduce the electric field. This property of electrical insulating materials is called the dielectric constant of the dielectric.
Types of dielectrics
Dielectrics, or insulators, are those bodies through which electric charges cannot pass from a charged body to an uncharged one. This property of dielectrics is due to the fact that under certain conditions there are no free charge carriers in them. If conditions change, such as when heated, free charge carriers may appear in the dielectric and it will begin to conduct electricity. So, the division of substances into conductors and dielectrics is conditional.
Dielectrics include all gases under normal conditions, liquids (kerosene, alcohols, acetone, distilled water, etc.), solids (glass, plastics, dry wood, paper, rubber, etc.).
In dielectrics, electric charges cannot move under the influence of an electric field throughout the entire volume of the body in the same way as free charges of a conductor.
Dielectrics are divided into two types:
Characteristics and physical properties of materials
The parameters of the conductors determine the scope of their application. Main physical characteristics:
- electrical resistivity - characterizes the ability of a substance to prevent the passage of electric current;
- temperature coefficient of resistance - a value characterizing the change in indicator depending on temperature;
- thermal conductivity - the amount of heat passing per unit time through a layer of material;
- contact potential difference - occurs when two dissimilar metals come into contact, used in thermocouples to measure temperature;
- temporary tensile strength and tensile elongation depend on the type of metal.
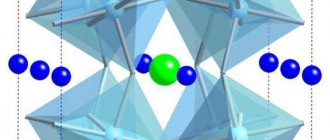
Types and types of dielectrics
The classification of dielectrics is quite extensive. There are liquid, solid and gaseous substances here. They are further divided according to certain characteristics. Below is a conditional classification of dielectrics with examples in list form.
- gaseous
- - polar
- - non-polar (air, SF6 gas)
- - polar (water, ammonia)
- - liquid crystals
- - centrosymmetric
- - amorphous
- - resins, bitumen (epoxy resin)
- - glass
- - disordered polymers
- – irregular crystals
- - ceramics
- - ordered polymers
- - glass-ceramics
- - molecular
- - covalent
- - ionic
- — displacement paraelectrics
- — paraelectrics “order-disorder”
- — single crystals
- - pyroelectrics
- — bias ferroelectrics
- — ferroelectrics “order-disorder”
- — linear pyroelectrics
- - with hydrogen bonds
- - covalent
- - ionic
- - electronic defects
- — ionic defects
- - polar molecules
- — macrodipoles
- — ferroelectric domains
- - crystals in the matrix
If we take liquid and gaseous dielectrics, then the main classification lies in the issue of polarity. The difference is in the symmetry of the molecules. In polar molecules the molecules are asymmetrical, in nonpolar ones they are symmetrical. Asymmetrical molecules are called dipoles. Polar liquids have such high conductivity that they cannot be used as insulating substances. Therefore, non-polar, also transformer oil, is used for these purposes. And the presence of polar impurities, even in hundredths, significantly reduces the breakdown level and negatively affects the insulating properties of non-polar dielectrics.
crystals are a cross between a liquid and a crystal, as the name suggests.
Another popular question about the properties and applications of liquid dielectrics is the following: is water a dielectric or a conductor? Pure distilled water contains no impurities that could cause current to flow. Pure water can be created in laboratory and industrial conditions. These conditions are complex and difficult to meet for an ordinary person. There is an easy way to check whether distilled water conducts current.
Create an electrical circuit (current source - wire - water - wire - light bulb - another wire - current source), in which one of the areas for current flow will be a vessel with distilled water. When the circuit is turned on, the light bulb will not light up - therefore no current flows. Well, if it lights up, it means water with impurities.
Therefore, any water that we encounter: from a tap, in a lake, in a bathroom - will be a conductor due to impurities that create the opportunity for current to flow. Do not swim in a thunderstorm or handle electricity with wet hands. Although pure distilled water is a polar dielectric.
For solid dielectrics, classification mainly lies in the question of activity and passivity or something. If the properties are constant, then the dielectric is used as an insulating material, that is, it is passive. If the properties change depending on external influences (heat, pressure), then this dielectric is used for other purposes. Paper is a dielectric; if water is saturated with water, then current is conducted and it is a conductor; if paper is saturated with transformer oil, then it is a dielectric.
Foil is a thin metal plate; metal is known to be a conductor. For example, there is PVC foil on sale, here the word foil is for clarity, and the word PVC is for understanding the meaning - after all, PVC is a dielectric. Although on Wikipedia, foil is a thin sheet of metal.
Amorphous liquids
- this includes resin, glass, bitumen, and wax. As the temperature rises, this dielectric melts, these are frozen substances - these are wild definitions that characterize only one facet of the truth.
Polycrystals
- these are, as it were, fused crystals united into one crystal. For example, salt.
Monocrystal
- this is a solid crystal, unlike the above-mentioned polycrystal, which has a continuous crystal lattice.
Piezoelectrics
- dielectrics in which, under mechanical action (tension-compression), an ionization process occurs. Used in lighters, detonators, ultrasound examinations.
Pyroelectrics
— when the temperature changes, spontaneous polarization occurs in these dielectrics. It also occurs under mechanical influence, that is, pyroelectrics are also piezoelectrics, but not vice versa. Examples are amber and tourmaline.
Types and classification of dielectric materials
Insulators are divided into groups according to several criteria.
Classification according to the state of aggregation of a substance:
- hard - glass, ceramics, asbestos;
- liquid - vegetable and synthetic oils, paraffin, liquefied gas, synthetic dielectrics (silicon and organofluorine compounds freon, freon);
- gaseous - air, nitrogen, hydrogen.
Dielectrics can be of natural or artificial origin, organic or synthetic in nature.
Organic natural insulating materials include vegetable oils, cellulose, and rubber. They are characterized by low heat and moisture resistance and rapid aging. Synthetic organic materials - various types of plastic.
Inorganic dielectrics of natural origin include: mica, asbestos, muscovite, phlogopite. The substances are resistant to chemical attack and can withstand high temperatures. Artificial inorganic dielectric materials - glass, porcelain, ceramics.
Effect of electric field on conductors, semiconductors and dielectrics.
Source - generator In modern generators usually:
the phase windings are located in the stationary part of the generator - the stator, and the magnetic field is created by a rotor rotating at the same speed, which is an electromagnet.
13. Electrical conductivity of conductors, dielectrics and semiconductors.
Depending on the size of the band gap1, many parameters of substances and, above all, electrical conductivity change sharply.
Conductors. The forbidden zone is equal to or close to zero. Electrons, due to their own thermal energy, can move to free levels and increase the conductivity of the substance. Typical conductors are metals.
Dielectrics. The band gap exceeds several electron volts. To transfer electrons from the valence band to the conduction band, significant energy is required, which can destroy the structure of the substance. Dielectrics have high resistivity.
Semiconductors. Semiconductors occupy an intermediate position in terms of band gap
. The bandgap value is 0.1-3 eV (silicon, germanium, etc.). In semiconductors, electrons can be easily transferred from the valence band to the conduction band using external energy (for example, increasing the temperature).
The division of solids into semiconductors and dielectrics is quite arbitrary. For example, dielectrics begin to conduct current at very high temperatures.
If a solid requires an energy of more than 6 eV to overcome the forbidden layer, then it is a dielectric, and if it is less than 6 eV, it is a semiconductor. The widest class of semiconductors, as shown by theory and experience, has a band gap of less than 2 eV.
14. The principle of operation of galvanic cells.
If two electrodes made of metals selected in a certain way are immersed in a liquid electrolyte, then on one of them, as a result of chemical reactions, an excess of electrons will appear (“–”), and on the other – a deficiency (“+”).
An electromotive force will act between the electrodes, and, therefore, the entire electrode-electrolyte system will turn into a chemical generator of electric current.
The first chemical current source is a galvanic cell made of copper and zinc plates immersed in a solution of table salt or sulfuric acid.
Primary galvanic cells cannot be returned to working condition after their filler (active substance) has been used up once. For such elements it is impossible, or at least uneconomical, to reverse the electrode process by passing current in the opposite direction. This type is usually simply called an element.
Secondary galvanic cells or batteries can be regenerated after depletion if current is passed through them in the opposite direction (charged), because the current generation processes occurring at their electrodes are, to a good approximation, electrochemically reversible.
The two most common types of batteries are acid (lead) and alkaline.
There is no fundamental difference between primary and secondary elements.
15. Acid and alkaline batteries.
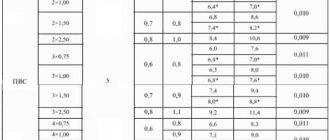
Acidic. The anode of a charged lead-acid battery is made of lead, and the cathode is made of lead dioxide. The metallic type of conductivity of PbO2 makes it suitable for use as an electrode. The electrolyte is a solution of H2SO4 (32–39%), in which PbSO4 and PbO2 are slightly soluble.
When charging, a reverse reaction occurs and the electrodes change their functions: the cathode becomes the anode, and the anode becomes the cathode. The emf of a lead battery depends on the ratio of the activity of acid and water:
As the battery operates, the acid concentration drops, and therefore the emf drops. When the EMF reaches 1.85 V, the battery is considered discharged. At a lower EMF, the plates are covered with a thin layer of PbSO4 and the battery is discharged irreversibly. To avoid this, the battery is periodically recharged.
Alkaline. In a charged alkaline iron-nickel battery, the anode is iron, the cathode is nickel (III) hydroxide, and the electrolyte is a 20% KOH solution. When the battery operates, iron oxidation occurs at the anode. at the cathode – reduction of nickel (III) hydroxide. The emf of an alkaline battery does not depend on the alkali concentration, since the expression under the logarithm sign includes constant values. Alkaline cadmium-nickel and silver-zinc batteries work similarly.
16. Methods of ionization of gases. Types of gas discharges.
Under normal conditions, gases are good dielectrics.
The electrical properties of gases are primarily related to the ionization of molecules or atoms.
Methods of ionization of molecules and atoms:
1. Heating. At temperatures starting from several thousand degrees, any gas is partially ionized and turns into plasma .
Plasma is a fully or partially ionized gas.
2. Exposure to electromagnetic radiation:
• ultraviolet radiation;
• X-ray radiation;
• gamma radiation.
3. Exposure to charged particles: alpha, beta particles, cosmic radiation.
4. Electronic shock
In an electrical discharge, a moving electron collides with a neutral atom and knocks out one or more atomic electrons, causing the neutral atom to become a positive ion and new electrons appear in the gas.
5. Electron capture. During ionization, some of the resulting electrons can be captured by other neutral atoms, and then negative ions will also appear.
Gas discharge is the process of passing electric current through a gas.
Non-self-sustaining gas discharge is a discharge in which the electrical conductivity of the gas is created by external ionizers. With the cessation of the action of external ionizers, the non-self-sustained discharge ceases. A non-self-sustaining gas discharge is not accompanied by gas glow.
Self-sustaining gas discharge is a discharge that persists after the external ionizer ceases to operate. Creation of an independent category. If, after reaching saturation, we continue to increase the potential difference between the electrodes, then the electrons acquire energy sufficient to ionize neutral atoms. The resulting electrons also acquire sufficient energy to ionize the remaining neutral molecules. Impact ionization occurs - the formation of an avalanche of electrons. The current strength increases hundreds and thousands of times. But ionization by electron impact alone cannot ensure the maintenance of an independent charge.
1. A glow discharge can be obtained at any pressure up to atmospheric pressure, however, most studies have been carried out at pressures from hundredths to several millimeters of mercury.
A distinctive feature of a glow discharge is a low current density at the cathode and a large (on the order of hundreds of volts) cathode potential drop.
The emission of electrons from a cold cathode occurs due to impacts of positive ions and fast atoms on the cathode, as well as for some other reasons (photoelectric effect, etc.).
2. Corona discharge occurs at normal pressure in a gas located in a highly inhomogeneous electric field (near the tips or wires of high voltage lines).
Ionization of the gas and its glow occur only near the electrodes.
Corona discharge is a harmful phenomenon accompanied by current leakage and loss of electrical energy.
To reduce corona damage, the radius of curvature of the conductors is increased, and their surface is made as smooth as possible.
During a thunderstorm, a corona discharge can form on sharp objects.
At a sufficiently high voltage between the electrodes, the corona discharge turns into a spark discharge
3. Spark discharge is an intermittent form of electric discharge in gases that occurs at atmospheric pressure when the gas is ionized along the entire length of the interelectrode space.
Gas ionization occurs not throughout the entire volume, but through individual brightly glowing channels, the so-called spark channels.
External manifestation: the release of a large amount of heat, a bright glow of gas, crackling or thunder.
The reasons for these phenomena: electron and ion avalanches, which lead to a huge increase in pressure (up to 107 -108 Pa) and an increase in temperature up to 10,000 °C.
An example of a spark discharge: lightning (diameter from 10 to 25 cm, length several kilometers, current strength tens and hundreds of thousands of amperes).
4. Cause of arc discharge : Intense emission of thermionic electrons from a hot cathode. These electrons are accelerated by the electric field and produce impact ionization of gas molecules.
Arc discharge characteristics:
• Cathode temperature - 3000 °C.
• Electrons bombard the anode, creating a depression (crater) in it.
• The gas temperature in the arc discharge channel reaches 5000-6000 °C.
The arc discharge was discovered by V.V. Petrov in 1802.
In 1876, P. N. Yablochkov was the first to use an electric arc as a light source.
Application: 1). arc furnaces for smelting steel, cast iron, bronze, etc.
2). for cutting and welding metal
5. Plasma is a partially or fully ionized gas in which the densities of positive and negative charges are almost equal.
Plasma as a whole is an electrically neutral system.
Types of plasma
Depending on the degree of ionization, plasma is divided into:
• weakly ionized (fractions of percent) - upper layers of the atmosphere - ionosphere
• partially ionized (several percent)
• completely ionized (a is close to 100%) – the sun, stars.
Plasma is the fourth state of matter.
Properties
Plasma particles move easily under the influence of electric and magnetic fields, because Coulomb forces act between charged plasma particles and decrease relatively slowly with distance.
Various types of oscillations and waves are easily excited in plasma.
Application
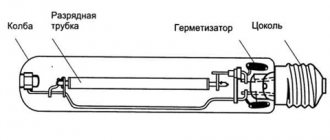
Gas discharge lamps
Gas lasers are quantum light sources.
17. Electric current in a vacuum.
Vacuum is a degree of rarefaction of a gas at which there are practically no collisions of molecules (pressure less than 10-13 mm Hg).
Electric current is impossible in a vacuum, because there are no free charge carriers in it.
To obtain an electric current in a vacuum, the presence of free charge carriers is necessary.
They can be obtained by emitting electrons from bodies in a vacuum.
This phenomenon is called electron emission.
18. Types of electronic emission.
· Thermionic emission is the emission of electrons by heated bodies. In metals, the concentration of free electrons is high, so even at average temperatures some electrons overcome the potential barrier at the metal boundary. With increasing temperature, the number of such electrons increases. The higher the temperature of the metal, the higher the density of the electron cloud. Under normal conditions, the number of electrons that left the electrode is equal to the number of electrons that returned to it (since the electrode becomes positively charged when electrons are lost). The operation of many vacuum electronic devices is based on the phenomenon of thermionic emission.
· Photoelectron emission
Photoemission is a phenomenon in which electrons are emitted from metals and semiconductors in a vacuum when irradiated with light. Photoelectron emission is a special case of the photoelectric effect.
Types of photo effect:
External photoelectric effect – photoemission.
The internal photoelectric effect is the redistribution of electrons among energy states in solid and liquid semiconductors and dielectrics, which occurs under the influence of radiation.
Gate photoelectric effect is the occurrence of EMF when illuminating two different semiconductors or a semiconductor and a metal (a type of internal photoelectric effect).
Nuclear photoelectric effect is the absorption of a gamma quantum (electromagnetic radiation quantum) by a nucleus and the decay of a compound nucleus with the emission of nucleons (usually neutrons).
Photoelectric effect is the emission of electrons by a substance under the influence of any electromagnetic radiation.
A theoretical explanation of the laws of the photoelectric effect was given in 1905 by Einstein.
Energy of the incident photon (quantum) hν
is spent on the electron performing work function
A
and on imparting kinetic energy to the photoelectron.
From Einstein's equation it follows that there is a minimum photon energy at which the photoelectric effect is possible.
· Secondary electron emission. Secondary electron emission is the emission of electrons by the surface of a metal, semiconductor or dielectric when bombarded with a beam of electrons.
The secondary electron current consists of:
electrons reflected by the surface (elastically and inelastically reflected electrons), and
“true” secondary electrons - electrons knocked out of the metal by primary electrons.
The secondary electron emission coefficient δ is the ratio of the number of secondary electrons to the number of primary ones.
Coefficient δ depends on:
nature of the material;
bombarding particle energy
particle incidence angle.
The coefficient δ for semiconductors and dielectrics is several times higher than for metals (the high concentration of electrons in metals prevents secondary electrons from leaving the metal).
· Field electron (electrostatic) emission - emission of electrons from the surface of metals under the influence of a strong electric field.
This type of emission is observed in an evacuated tube in which the cathode is the tip and the anode is the inner surface of the tube.
19. Laws of external photoelectric effect.
1st law: each quantum absorbs only one electron. Therefore, the number of ejected photoelectrons is proportional to the light intensity.
2nd law: the maximum kinetic energy of electrons ejected by light increases linearly with the frequency of light and does not depend on its intensity (see Einstein’s equation).
3rd law: with a decrease in light intensity, the kinetic energy of photoelectrons decreases, therefore, at some sufficiently low frequency, the kinetic energy of photoelectrons will become zero and the photoelectric effect will stop.
The “red limit” of the photoelectric effect depends only on the work function of the electron, i.e. on the chemical nature of the substance and the state of its surface (Na – 500 nm, Zn – 372 nm, Ag – 260 nm, Pt – 196 nm)
20. Magnetic induction. Ampere's law
Magnetic induction is the influence of a magnet on an object without mechanical contact (the main characteristic of a magnetic field).
In any body there are two types of currents:
• macroscopic (free electrons in metals, etc.);
• microscopic (molecular currents).
The magnetic field of macrocurrents is described by the magnetic field strength vector H.
Property of microscopic currents: they are able to rotate in the magnetic fields of macroscopic currents, creating an additional magnetic field.
Ampere's law. The force acting on a conductor carrying current is equal to:
where l
– length of the conductor;
I
– current strength;
α – angle between the direction of the current and the magnetic induction vector
⇐ Previous2Next ⇒
Recommended pages:
Why dielectrics do not conduct electricity
Low conductivity is due to the structure of dielectric molecules. Particles of matter are closely connected to each other, cannot leave the confines of the atom and move throughout the entire volume of the material. Under the influence of an electric field, the particles of an atom can become slightly looser and become polarized.
Depending on the polarization mechanism, dielectric materials are divided into:
- non-polar - substances in different states of aggregation with electronic polarization (inert gases, hydrogen, polystyrene, benzene);
- polar - have dipole-relaxation and electronic polarization (various resins, cellulose, water);
- ionic - solid dielectrics of inorganic origin (glass, ceramics).
EXAMPLES OF TASKS
Part 1
1. A lightweight, uncharged ball of metal foil is suspended on a thin silk thread. When a rod with a positive electrical charge is brought to the ball (without touching), the ball
1) repels from the rod 2) experiences neither attraction nor repulsion 3) is attracted to the rod at large distances, repelled at short distances 4) is attracted to the rod
2. A positively charged glass rod was brought, without touching, to an uncharged light metal cartridge suspended on a silk thread. Which figure correctly shows the behavior of the cartridge case and the distribution of charges on it?
3. A positively charged rod was brought to an uncharged electrometer. What charge will the ball and needle of the electrometer acquire?
1) the ball and arrow will be negatively charged 2) the ball and arrow will be positively charged 3) the ball will have an excess positive charge, the arrow will have an excess negative charge 4) the ball will have an excess negative charge, and the arrow will have an excess positive charge
4. A positively charged glass rod is brought to two identical charged balls suspended on insulating threads. As a result, the position of the balls changes as shown in the figure (the dotted lines indicate the initial position of the threads). It means that
1) both balls are positively charged 2) both balls are negatively charged 3) the first ball is positively charged and the second is negatively charged 4) the first ball is negatively charged and the second is positively charged
5. Ball B was brought to a negatively charged ball A suspended on a thin thread without touching it. Ball A deviated as shown in the figure. Sharik B
1) has a negative charge 2) has a positive charge 3) may not be charged 4) may have both a positive and negative charge
6. A dielectric rod was brought to the negatively charged electroscope without touching it. In this case, the leaves of the electroscope diverged to a noticeably larger angle. The charge of the stick can be
1) only positive 2) only negative 3) both positive and negative 4) equal to zero
7. An insulated negatively charged metal ball was brought close to the uncharged insulated conductor AB. As a result, the leaves suspended on both sides of the conductor diverged at a certain angle (see figure).
The charge distribution in the AB conductor is correctly shown in the figure.
8. An uncharged metal ball is suspended from a thread. A charged stick was brought to him from below. Will the thread tension change, and if so, how?
1) will not change 2) will increase regardless of the sign of the charge of the stick 3) will decrease regardless of the sign of the charge of the stick 4) will increase or decrease depending on the sign of the charge of the stick
9. What material can be used to make the rod connecting the electroscopes shown in the figure?
A. Steel B. Glass
1) only A 2) only B 3) both A and B 4) neither A nor B
10. Two metal balls mounted on an insulating stand were connected by a metal rod. A negatively charged stick was brought to the right ball, then the rod and charged stick were removed. What charge will be on the right and left balls?
1) on the right ball - positive, on the left - negative 2) on the right ball - negative, on the left - positive 3) on the right and left balls - positive 4) on the right and left balls - negative
11. From the list of statements below, select two correct ones and write their numbers in the table.
1) There is an electric field around an electric charge. 2) In a dielectric placed in an electric field, a redistribution of charges occurs. 3) The electric field is invisible and cannot be detected. 4) During electrification through influence, a redistribution of charges occurs in the conductor. 5) A dielectric can be given an electric charge by placing it in an electric field.
12. An electrometer with a ball at its end is placed in a field of negative charge. At the same time, its arrow deviated by a certain angle. How did the number of charged particles in the electrometer change? Establish a correspondence between physical quantities and their possible changes in this case. Write down the selected numbers in the table under the corresponding letters. The numbers in the answer may be repeated.
PHYSICAL QUANTITY A) number of protons on the ball B) number of electrons on the ball C) number of electrons on the arrow
NATURE OF CHANGE 1) increased 2) decreased 3) did not change