What are conductors and dielectrics
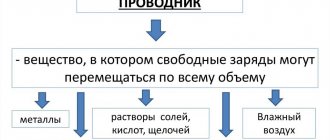
Conductors are substances that have in their structure a mass of free electrical charges that can move under the influence of external force throughout the entire volume of the material.
The group of conductors in an electrostatic field includes metals and their compounds, some types of electrical coal, solutions of salts (acids, alkalis), and ionized gases.
The best conductive material is considered to be metal, for example gold, platinum, copper, aluminum. Non-metallic substances that conduct current include carbon.
Conductor
Dielectrics are substances whose properties are opposite to conductors. In the absence of heating, charged particles in a neutral atom are closely interconnected and cannot move within the bulk of the material. In this regard, electric current cannot flow in a non-conductor.
Dielectric
Materials that do not conduct electric current include: ceramics, rubber, paper, glass, porcelain, resin, dry wood. Gas is considered the best dielectric. The qualities of dielectrics depend on the temperature and humidity of the environment in which they are located.
Important! As humidity increases, dielectrics may lose their non-conducting properties.
Conductors and dielectrics are actively used in the electrical field. Example - the material from which wires (cables) are made are conductors made of metal. Insulating shells for them are made from dielectrics - polymers.
Properties of materials
The best conductors are those made from silver, gold or platinum. Their widespread use is limited only by the high cost of the material. Such products have found application in the defense and space industries. In these areas, it is important to ensure the highest quality equipment, regardless of cost.
The scope of application of copper and aluminum materials is much wider. Low cost and excellent conductive qualities have made it possible to use them in many industries.
In dielectrics, an increase in temperature can lead to the appearance of free electrical charges. These are electrons that have been detached from the nucleus due to temperature fluctuations. Usually this is a small amount of free charges. But there are insulators in which this number reaches significant sizes. In this case, the insulating qualities of the dielectric deteriorate.
Note! A dielectric is considered reliable if the small leakage current that occurs in it does not interfere with the operation of the entire system.
The best dielectric is absolute vacuum, as well as completely purified water. But they cannot be found in nature, and it is very difficult to create them artificially. The inclusion of any impurity in a liquid provides it with conducting qualities.
Content
In the last lesson we already mentioned conductors and dielectrics. We defined them as substances in which free electrons are present or absent. They are the ones who carry out the transfer of electrical charge. Conductors have them, but dielectrics do not.
And yet, the main feature that we will consider is the ability to conduct current or transmit electric charge. Based on this ability, substances are divided into three main classes: conductors, semiconductors and dielectrics . In this lesson we will define each class of substances, consider the nature of semiconductors, which we have not encountered before.
Properties of conductors
Resistivity
The main characteristics of electrical conductors are:
- resistance,
- electrical conductivity.
When electrons move through a conducting substance, they collide with ions and atoms. This leads to resistance.
If a potential difference is created between two conductors, then an electric current will flow through the third one connecting them. The direction of its movement will be from greater potential to less. In this case, the carriers will be electrons that are not connected to each other, which determine the value of the electrical conductivity of the substance.
Electrical conductivity is the ability of a material to pass electric current. This indicator is inversely proportional to the resistance of the material, measured in siemens, see.
Depending on the charge carriers, electrical conductivity can be:
- electronic,
- ionic,
- hole
Conductor with electronic conductivity
Note! A reliable conductor is characterized by low resistance to the flow of moving electrons and, accordingly, high electrical conductivity. The greatest conductivity is the property of the best conductor.
The selection of conductive materials should be carried out in accordance with their properties:
- Electrical (resistivity and temperature coefficient of resistance);
- Physical (melting degree, density);
- Mechanical (resistance to stretching, bending, machine processing);
- Chemical (interaction with the environment, the possibility of connection during welding, soldering).
Metals without impurities have low resistivity. For alloys this figure increases. Resistance also increases with increasing temperature.
Important! When cooled to critical values, the resistance of most conductive substances drops to zero. This property is called superconductivity.
When choosing conductors for electrical installations, power lines, protective grounding and other applications, it is important to take into account all the qualities of the materials.
Summary
- In conducting materials, the outer electrons in each atom can easily come or go and are called free electrons.
- In dielectric (insulating) materials, outer electrons do not move as freely.
- All metals conduct electricity.
- Dynamic electricity, or electric current, is the uniform movement of electrons along a conductor.
- Static electricity is a stationary (if it is on a dielectric), accumulated charge formed by an excess or deficiency of electrons in an object. It is usually formed by charge separation through contact and separation of dissimilar materials.
- In order for electrons to flow continuously (endlessly) through a conductor, there must be a complete, uninterrupted path along which they can move both in and out of that conductor.
Original article:
- Conductors, Insulators, and Electron Flow
Dependence of conductor resistance on current frequency
When exposed to electric current, the induction of a magnetic field occurs inside a straight conductor and in the space surrounding it. Magnetic lines form concentric circles.
Alternating current distribution across the cross section
How to calculate amperes
If a current-carrying conductor is conditionally divided into several current filaments parallel to each other, then it can be established that the closer the current filament is to the axis of the conductor, the greater the magnetic flux that closes inside it covers it. The inductance of the filament and the inductive reactance are proportional to the magnetic flux associated with it.
In this regard, in threads with alternating current located inside the conductive substance, greater inductive reactance occurs than in threads located outside. An uneven current across the cross-section is formed, increasing from the axis to the surface of the conductor, which explains the increase in the resistance of the conductors to alternating current. This phenomenon is called the skin effect.
Due to the uneven distribution of current density, the resistance of the conductor increases. At a low frequency of 50 Hz and a small cross-section of copper wire, the phenomenon of surface effect is almost imperceptible. With a significant increase in the frequency and cross-section of the iron conductor, this phenomenon will be more active.
Note! The higher the frequency of the current in the circuit, the closer to the surface of the conductor the electrical charges are, and the more its resistance increases.
Among the list of conductor materials with such properties as high conductivity are aluminum, copper and some alloys: phosphor bronze, brass and many others. These materials have found wide application in the manufacture of coils used in electrical machines, apparatus and instruments. The greater the mechanical strength and the lower the resistivity of the conductor, the better it is considered. Depending on the use case, the requirements for these properties vary. For example, a cable stretched between the poles of a power line or contact network requires a conductor with a certain mechanical tensile strength. And when creating a coil of electric machines or devices, you need a conductor with the lowest possible resistivity value, including if this will reduce the mechanical strength.In order to achieve the lowest resistivity, the metal must be absolutely free of impurities. Because the resistivity indicator increases with the addition of any impurity. Even if another metal with a lower resistivity is added to the base metal, the overall resistance will still increase. This happens due to the fact that if you add any, even the smallest amount of impurity, the crystal lattice of the metal is distorted. In addition, this also manifests itself during mechanical deformation. Therefore, when metals are processed by pressure, their resistivity increases. The same effect occurs in the production of wire for wires.
Brass and copper are used in the manufacture of various current-conducting parts. Copper wires are produced by rolling or drawing. The product has the properties of high mechanical strength and hardness. This copper is grade MP. It is used in the production of wires without insulation, distribution busbars, collector plates and others. If hard-drawn copper is put through heat treatment with an annealing temperature of 330-350 °C, you will get soft MM grade copper. This product is flexible and capable of significant stretching. This occurs due to the fact that during such heating the copper structure recrystallizes, and the internal stresses that appeared during broaching disappear. The material becomes “viscous”, and the electrical conductivity also increases. This copper is used in the production of cables, insulated wires, etc.
Bronze (an alloy of copper with other metals) is also used as a conductor. Bronze has great mechanical strength and high resistivity. Cadmium bronze is used in the production of plates for collectors and cables. Beryllium bronze is used for springs, brush holders, sliding contacts, and switch knives. Brass, with the same properties, resists abrasion well and is convenient for stamping and drawing. It can be soldered and welded.
The next most popular conductor is aluminum. When drawn, the finished product comes out quite hard, and during subsequent heat treatment it becomes flexible. The technology of adding elements such as copper, manganese, silicon and magnesium to an aluminum alloy in order to increase strength and mechanical properties has become widespread. As a result of such additions, various aluminum alloys are obtained, such as duralumin, silumin and others.
There are two types of aluminum hardness: AT - hard aluminum and AM - soft aluminum. Wires and other aluminum parts at the joints are fastened using rivets and welding, because soldering aluminum using the usual method is difficult due to the presence of aluminum oxide on the surface of the parts. Its melting point is approximately 2000 °C.
In our story about the properties of the material, we went into theory, but from a practical point of view, these materials are in no way amenable to professional video shooting, even with the most powerful professional video camera, since the particles that make up the material are too small. Even professional videographers will not be able to film particles using professional equipment using powerful spotlights - light sources. But professional video shooting is perfect and will be appropriate for celebrations and other festive events.
Formula for determining conductor length
Ampere - what is it?
You can find the length of the conductor by directly measuring it, for example, with a tape measure. If you need to calculate the length of hidden electrical wiring in a home, you need to take into account that it is usually laid horizontally along the walls at a distance of 15-20 cm from the ceiling. Vertically, at right angles, make drops on switches and sockets. If the conductor is difficult to reach (grounding conductors) or is long, this method may be difficult to implement.
Then the length of the conductor is determined in a different way. To do this you need to prepare:
- construction tape,
- tester,
- calipers,
- table of electrical conductivity of metals.
First you need to measure the resistance of individual sections of electrical wiring. Next, determine the cross-section of the wire and the material from which it is made. Typically, aluminum or copper conductive materials are used in everyday life.
From the formula for determining resistance ( R = r * L * s), find the length of the conductor using the formula:
L = R / r*s,
Where:
- L – wire length,
- R is its resistance,
- r – resistivity of the material (for copper it ranges from 0.0154 to 0.0174 Ohm, for aluminum – from 0.0262 to 0.0278 Ohm),
- s – cross-sectional area of the wire.
Calculate the wire cross-section:
S = π/4 * D2,
Where:
- π – a number approximately equal to 3.14;
- D is the diameter measured with a caliper.
If you need to find the length of a wire wound into a coil, determine the length of one turn in meters and multiply by the number of turns.
If the coil is round, measure its diameter, multiply by the number π and the number of turns:
L = d * π * n,
Where:
- d – coil diameter,
- n – number of turns of wire.
Types of conductors
The state of electrically conductive materials can be solid, liquid, or gaseous.
Solids are groups of metals, their alloys and some modifications of carbon. Metals conduct heat and electricity well.
Liquids are molten metals and electrolytes. At low temperatures, the liquid conductor can be mercury or gallium. The melting point of the remaining elements is too high.
The flow of current through a metal, whether in a solid or liquid state, occurs through the movement of free electrons. Due to this, its electrical conductivity is called electronic, and the substance itself is called a conductor of the first kind.
A conductor of the second type (electrolyte) is an acidic, alkaline, saline solution and a melt of ionic compounds. Molecules (ions) are transported in it simultaneously with the movement of current, so over time the structure of the electrolyte changes, and the electrolysis product is deposited on the electrodes.
In a low-intensity electric field, any gas or vapor does not conduct current. But if the voltage reaches its maximum critical point, when impact and photo-ionization begin, the gas can become a conductor with electronic and ionic conductivity. When there are equal numbers of electrons and positive ions per unit volume, the highly ionized gas will become a balanced, electrically conductive substance called plasma.
So let's start with the conductor
A conductor is matter that consists of free carriers of charged particles. When these particles move, thermal energy is generated, which is why they gave it the name thermal motion.
There are two main parameters of a conductor - resistance, denoted by the letter R, or conductivity, denoted by the letter G. Conductivity is the opposite indicator of resistance - G = 1/R.
That is, a conductor is a material that conducts current.
What is a conductor? Metals are the best conductors, especially copper and aluminum. Also conductors are salt solutions, wet soil, and carbon. The latter has found wide application in working with sliding bonds.
An example of such an application is brushes in an electric motor. The human body is also a conductor of electric current. But the electrical conductivity properties of the above materials are still lower than those of metals.
The very structure of metals contains a huge amount of free charged particles, which makes them the best conductors.
When a metal is exposed to electric fields, a process called electrical induction occurs. That is, charged particles begin to actively move and distribute.
Properties of dielectrics
The choice of dielectrics should be made in accordance with their properties:
- Electrical: breakdown voltage (at which breakdown occurs), electrical strength (field strength at which breakdown occurs);
- Physico-chemical: resistance to heat (the ability to withstand operating temperatures for a long time), cold resistance (the ability to withstand temperature changes), wettability (the ability to reject moisture);
- Chemical: resistance to aggressive environments, solubility in varnishes, possibility of gluing;
- Mechanical: radiation resistance, viscosity (for liquid dielectrics), corrosion protection, tensile strength, possibility of tooling.
What is a semiconductor
By designation, a semiconductor is a substance whose electrical conductivity is less than that of a metal and greater than that of a dielectric.
Semiconductors
The difference between a semiconductor is that its electrical conductivity depends on the temperature regime and the volume of impurities in the composition. The material has both conductive and dielectric characteristics.
As the temperature increases, the electrical conductivity of a substance increases, and the resistance level decreases. As the temperature decreases, the resistance tends to infinity.
Note! When the temperature reaches zero, the semiconductor behaves as an insulator.
Due to their unique properties, semiconductors are used in many industries: low-power SMD on printed circuit boards, and high-power devices, for example, thyristors in power converter technology.
Zone theory
The band theory of solids is the theory of the movement of valence electrons in the potential field of a crystal lattice. Quantum mechanics believes that free electrons can have any energy, the spectrum of which is continuous.
The electrons of isolated atoms have a certain discrete amount of energy. When individual atoms combine into molecules and form a substance, the electronic levels of the atom shift. Thus, from the energy levels of individual atoms in a solid, bands of energy level zones are formed.
The upper filled band, the valence band, corresponds to the energy level of the valence electrons of the outer shell. The closest to it, unfilled, is the conduction band. The relative position of both zones determines the processes occurring in a solid, and materials are classified into groups: conductors, semiconductors, dielectrics.
Zone classification
In conductors, the conduction band and valence band are combined. The resulting overlap zone allows the electron to move freely when receiving even small amounts of energy.
In semiconductors, the bands do not overlap. The distance between them, called the band gap, is less than 2.0 eV. At zero temperature, there are no electrons in the conduction band, and the valence band is filled with them. As the temperature increases, some electrons are thrown into the conduction band due to thermal motion. The semiconductor becomes electrically conductive.
In dielectrics, the zones, just like in semiconductors, do not overlap. The band gap here is more than 2.0 eV. In order to transfer electrons from the valence zone to the conduction zone, it is necessary to significantly increase the temperature. At low degrees, no electric current is conducted.
Superconductivity
The property of a material to have zero electrical resistance at a temperature below a certain value is called superconductivity.
For some conductive substances, this ability occurs at cold temperatures close to the chemical state of liquid helium.
In 1986, substances with high-temperature superconductivity were discovered. For example, ceramics made of oxygen, barium, copper, lanthanum do not conduct current under normal conditions, but due to heating they become a superconductor.
In practice, substances are used that transmit electric current at 58 degrees Kelvin or more, that is, at a temperature above the boiling point of nitrogen.
Solid high-temperature superconductors are most often used. Liquid and gaseous ones are used less frequently. All these materials are necessary for the manufacture of modern electrical devices of various capacities.
Insulators
Insulation tape
Insulators are materials that resist or block the passage of electrical current through them. You can also call them bad conductors. They do not provide a good path for electrical current because they do not contain a weakly bound electron in their atoms. Instead, their electrons are tightly packed into atoms.
Some materials have very good insulating properties, such as rubber and glass. While others have poor insulating properties such as water, damp wood, etc. Humans are also poor conductors and poor insulators. This means that when the voltage is high, we can easily get electrocuted (never touch 110V or 220V lines).
Insulators are mainly used to insulate conductors. So if someone accidentally touches them, they won't be electrocuted.
Good Examples of Insulators
Rubber is a good insulator of electricity and heat.
We need good insulators to insulate electrical wires and heat protection devices such as refrigerators. Good insulators provide very high resistance to electrical current so that it cannot pass through them. Below is a list of famous insulators that are used to insulate power lines, home wiring, houses in winter, etc.
● Porcelain
● Rubber
● Plastic
● Paper
● Glass