What is the galvanization process
Having found out what electroplating is, you can begin to study the important details. If a cathode is used for deposition, the anode is selected from the appropriate material. The principle of operation is gradual destruction to replenish the loss in the solution of working ingredients.
Electroplating at home
The composition of the medium must be selected in such a way as to minimize (completely eliminate) deterioration in the quality of the coating due to the presence of certain impurities. The following factors must also be taken into account:
- to increase the efficiency of useful physical and chemical processes, increasing temperature is useful;
- you will need a fairly powerful DC source;
- In order not to perform some actions manually, control and automation tools are needed.
Important! Since it is planned to organize production at home, it is necessary to pay special attention to safety issues.
Electrolytic electroforming
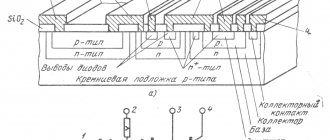
First of all, an imprint is taken from the object or product being copied, that is, a mold is made from fusible metal, wax, plasticine or plaster. The object to be copied, rubbed with soap, is placed in a cardboard box and filled with Wood's low-melting alloy or other low-melting alloys.
After casting, the object is removed and the resulting mold is degreased and subjected to copper plating in an electrolytic bath. In order to-
To prevent metal from being deposited on those sides of the mold where there is no imprint, they are coated with molten wax or paraffin using a brush. After copper plating, the low-melting metal is melted in boiling water to form a matrix. The matrix is filled with plaster or lead, and the copy is ready.
The following wax composition is used to make molds:
- Wax - 20th century. h.
- Paraffin - 3rd century. h.
- Graphite - 1st century. h.
If the mold is made of dielectric (wax, plasticine, paraffin, gypsum), its surface is covered with an electrically conductive layer. The conductive layer can be applied by reducing certain metals (silver, copper, nickel) or mechanically - by rubbing flake graphite into the surface of the mold with a soft hair brush.
Graphite is thoroughly ground in a porcelain mortar, sifted through a sieve or gauze and applied to the surface of the product with a soft brush or cotton swab. Graphite sticks better to plasticine. Forms made of plaster, wood, glass, plastic and papier-mâché are coated with a solution of wax in gasoline.
Graphite powder is applied to the surface that has not had time to dry, and excess, non-adherent graphite is blown off.
The electroplated coating is easily separated from the graphite-coated mold. If the mold is made of metal, then on its surface it is necessary to create an electrically conductive film of oxide, sulfide or other insoluble salt, for example, on silver - silver chloride, on lead - lead sulfite, so that the mold is well separated from the coating.
Copper, silver and lead surfaces are treated with a 1% solution of sodium sulfide, as a result of which insoluble sulfides are formed on them.
Deposition of metal on the surface of the mold. The prepared form is immersed in a bath, the circuit of which is energized so that the separating film does not dissolve. First, a “tightening” (coating) of a conductive layer of copper is carried out at a low current density in a solution of the following composition:
- Copper sulfate (copper sulfate) - 150-200 g,
- Sulfuric acid - 7-15 g,
- Ethyl alcohol - 30-50 ml,
- Water - 1000 ml.
The operating temperature of the electrolyte is 18-25° C, the current density is 1-2 A/dm^2. Alcohol is necessary to increase surface wettability. After the entire surface is “covered” with a layer of copper, the mold is transferred to an electrolyte intended for electroplating.
For galvanoplastic work (copper plating), the following composition is recommended:
- Copper sulfate (copper sulfate) - 340 v. h.
- Sulfuric acid - 2 c. h.
- Water - 1000 v, h.
Electrolyte temperature 25-28° C. Current density 5-8 A/dm2.
Purposes of Metal Electroplating
Capacitor energy
What is electroplating for household use? Theoretically, it is not too difficult to find a specialized enterprise, conclude an agreement, and receive a finished product with official guarantees. However, the practical implementation of such ideas is associated with various difficulties:
- payment for services and loss of time;
- lack of good specialists or relevant industries nearby;
- the reluctance of performers to reconfigure existing equipment to perform a relatively small amount of work.
Only you can create a unique electroplating with special characteristics yourself. Technology opens up vast opportunities for individual creativity. As will become clear after studying the data presented in the publication, the technology can be reproduced with high quality without excessive costs.
Galvanic coating provides an impeccable appearance for products with complex shapes
The photograph clearly demonstrates the excellent quality of processing of the smallest details and hard-to-reach areas. In addition to improving aesthetic parameters, metal electroplating helps create a layer of low electrical resistance on the insulator.
Stainless steel is expensive. Instead, the resistance of products at high humidity is increased with the help of copper plating. The technology is suitable for the production of spectacular jewelry, decorative and functional furniture elements. With its help, miniature parts are strengthened and chemical neutrality is ensured.
In cosmetology, low-intensity galvanic discharge is used to improve the functional state of the skin and remove individual defects.
Interior design and architecture
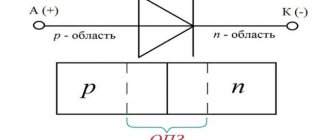
Galvanoplasty is a special method of forming a product of a certain shape from non-ferrous metal by depositing it in a molten state on a pre-prepared matrix. This process occurs under the influence of electric current. Thus, through the classical electrolysis procedure, it is possible to obtain metal copies of various objects.
Galvanoplasty (electroforming). Brooch (copper).
The thickness of the metal deposit deposited on the matrix during the electroforming process ranges from 0.25 mm to 2 mm. You can see that this layer of metal turns out to be quite thin, but it allows you to fully convey the shape of the future product in the smallest detail.
Products created by electroplating.
Electroplating is an effective technology for creating copies of relief originals by electrolytic copying. Today, electroplating continues to be the most popular and in-demand method of obtaining the most accurate samples of small art objects, despite the emergence of the latest three-dimensional scanning and 3D printing technologies.
Products using the electroforming technique
Electroforming technology
The technology of creating an exact copy of an object or piece of art using the electroplating method consists of several stages:
- Making a cast of a relief object from wax or other plastic material. In this case, it is necessary to take into account the fact that the surface of the copied product must have the property of conducting electric current. If the model is made of non-conductive materials, then any electrically conductive coating is applied to it in various ways. Often, crushed granite dust is rubbed into a wax sublayer or a method of chemical reduction of metals on the surface of the original is used.
- Placing the cast in electrolyte - the finished model is placed in a special container with an electrolyte solution.
- Carrying out an electrolysis procedure , during which, by passing current through a molten metal solution, a sufficiently thick layer of metal is built up on the surface of the impression, which evenly fills all the irregularities of the impression.
- Separation of the cast from the metal layer after the end of the electrolysis process. The copy is separated from the original by a pre-applied barrier layer or by chemical dissolution (melting) of the original.
Types of metals for electroforming
To make exact copies of objects or cover them with a thin metal layer, the following types of metals are used:
- Copper
- Nickel
- Chromium
- Silver
- Gold
- Iron
- Tin
- Rhodium
To this we can add that copper is most often used as an intermediate layer in the process of nickel plating, chrome plating, silver plating and gold plating. In addition, copper is often the only and main layer of metal in the electroforming process.
Scope of application
The electroplating method is used to manufacture metal parts of complex configurations. Such parts are difficult or even impossible to create using metal machining or conventional casting. Therefore, in such cases, the galvanoplastic method is the only way out.
However, the technology of galvanoplasty is most widespread in the manufacture of the following types of products:
- Artistic copies of sculptures
- Copies of bas-reliefs and high reliefs
- Figured dishes
- Jewelry
- Gramophone records
- Printing rollers
- Metal products with micron parameters
- Memorial plaques
- Memorial plaques
- Brand names
- Coins
- Coats of arms
- Medals
- Emblems
- Logos
- Decorative symbolism
- Busts
- Portraits
- Metal paintings
- Decorative panels
- Icons
- Settings for icons
- Crowns for icons
- Furniture inserts
- Flower pots
- Flower vases
- Openwork and decorative elements, ornaments and patterns
The electroplating method is widely used in restoration work or for creating interior items.
Electrotype
Electroplating equipment
Faraday cage
There is no need to “take away bread” from the owners of professional beauty salons. The corresponding techniques must be performed especially carefully so as not to cause harm to health. However, any ordinary person is able to prepare a high-quality set of equipment to solve technical problems.
The main component is a reliable and fairly powerful DC source. Adjustments in the desired range of voltage (1-12.5 volts) and current (up to 50-60 A) with a built-in indicator of the measuring device will be useful. The values of the required electrical parameters are selected after determining the operating settings of the technological operations.
A container with suitable dimensions is selected from a chemically neutral material. Heat-resistant plastic will do. However, it is better to use glass taking into account the following advantages:
- long-term preservation of consumer properties;
- strength, resistance to high temperatures;
- easy to clean.
Equipment set
As you can see in the photo, the electrodes can be fixed to the walls. The use of "crocodiles" speeds up the connection. To heat to the desired temperature, an electric stove with smooth power adjustment is useful. Scales are needed to accurately prepare the mixture.
Book
Electroplating is a technology for producing exact metal copies by depositing metal onto models, which are separated after the process is completed. The accuracy of the working dimensions and surface roughness of the resulting galvanoplastic copies depend entirely on the accuracy of the dimensions and surface roughness of the model on which metal is deposited.
An important role in the process of galvanoplastic formation of a product is played by preparing the surface of the mold used and creating a conductive layer on it.
Before applying the conductive layer, the surface of the model must be washed and degreased. The quality of degreasing is controlled visually.
Application of a conductive layer.
There are several types of conductive layers, each of them has its own advantages and disadvantages, and not all can be used in any situation. The choice of conductive layer depends on a number of factors, including the material of the model.
For models made of elastomers (rubbers, rubbers, etc.), colloidal graphite is most often used. The surface is pre-treated (wipe) with acetone or alcohol and dried. Graphite is applied with a soft brush to the surface of the model until the layer looks uniform and uniform. Excess graphite is blown off, after which the model is washed. This method is recommended to be used when it is possible to penetrate with a brush into all cavities of the matrix and evenly apply a layer of graphite.
For models with more complex surface topography, it is recommended to apply a conductive silver film. To do this, the model is degreased, washed and immersed in a sensitization solution for 5-10 minutes.
Composition of the sensitization solution:
| Electrolyte composition (g/l) and operating mode | Sensitization solution |
| Tin chloride | 10-30 |
| Hydrochloric acid, ml/l | 2-10 |
| Temperature, 0C | 18-25 |
After treatment in this solution, the model should be thoroughly washed in cold water, during which hydrolysis of tin dichloride occurs with the formation of poorly soluble compounds.
After sensitization, the process of chemical silvering is carried out from solutions:
A.
| Silver nitrate, g/l | 4 |
B.
| Pyragolol, g/l | 3,5 |
| Citric acid, g/l | 4 |
These solutions must be prepared in separate containers and cooled to a temperature of 1-15 0C, then, immediately before silvering, with stirring, solution “B” is poured into solution “A”, according to the following technology:
Pour solution “A” directly onto the model, and then, carefully stirring the solution with the model, while simultaneously diluting with distilled water, pour in solution “B”. Solution “A”, solution “B” and distilled water are taken in a ratio of 1:1:1. The operation must be repeated 2 times.
Next, the model with the applied conductive layer is immersed in a copper sulfate bath for tightening.
For models made of dielectrics , as a rule, a method of chemically applying a conductive layer is used. The model is first very thoroughly degreased, special attention is paid to such a parameter as “surface wettability”
Previously, separate solutions were often used to sensitize and activate the surface of the dielectric, but nowadays solutions of the “mixed” type are mainly used, in which sensitization and activation occur simultaneously.
Composition of the solution and operating mode:
| Electrolyte composition (g/l) and operating mode | Mixed sensitization solution |
| Palladium dichloride | 0,5-1 |
| Stannous chloride | 40-45 |
| Hydrochloric acid | 70-75 |
| Potassium chloride | 140-150 |
| Temperature, 0C | 15-25 |
After processing in a “mixed” solution, the model must be thoroughly rinsed in cold water, this is necessary to form a film of colloidal palladium on the surface of the model.
Next, the model can be hung in a sulfuric acid copper plating bath for further metallization.
Application of semiconductor films.
The essence of this method consists in the operation of sorption of inorganic substances by the surface of the polymer and their conversion into acid-soluble compounds under the influence of sulfonating agents. Let's consider the application of conductive films based on lead and copper sulfide.
Lead sulfide is applied from a solution of the following composition:
| Electrolyte composition and operating mode | Hot sulfidation solution |
| Lead nitrate, conc., ml/l | 50 |
| Potassium caustic, g/l | 4-5 |
| Thiourea, conc., ml/l | 30 |
| Temperature, 0C | 45-60 |
| Process time, min | 20-30 |
After applying the lead sulfide film, the model must be rinsed in hot running water; if there are uncovered areas, the operation must be repeated.
The disadvantage of this method is the increased temperature, which makes it difficult to work with some types of dielectrics, or with models whose size is precisely specified. Another disadvantage is the fact that this solution is essentially disposable.
Application of a conductive layer of copper sulfide.
The advantages of this method over the one described above are the short duration of the process and the relatively high stability of the solutions used. The technology for applying copper sulfide consists of sequentially treating the surface with a solution of a metal salt, water and a solution of a sulfiding agent. Adsorption on the surface of the hydrolysis products of the metal salt occurs at the stage of washing with water. Technology for applying conductive copper sulfide film:
- Sorption in solution:
- Hydrolysis in water for 0.1-0.2 minutes.
- Sulfidation in solution:
- Rinsing in water for 0.1-0.5 min
| Electrolyte composition and operating mode | Sorption solution |
| Copper sulfate, g/l | 10-100 |
| Zinc sulfate, g/l | 50-100 |
| Ammonia aqueous, ml/l | 150-200 |
| pH | 8,5-9,5 |
| Temperature, 0C | 18-25 |
| Process time, min | 0,5-1 |
| Electrolyte composition and operating mode | Sulfidation solution |
| Sodium sulfide, g/l | 10-50 |
| Temperature, 0C | 18-25 |
| Process time, min | 0,1-0,5 |
The model or part goes through the stages described above several times until a brown film appears on it, when the film becomes uniform over the area of the part, the process can be stopped and tightening with copper or nickel can begin.
Application of galvanic deposits.
After applying the conductive layer to the model, it is necessary to carry out the operation of “tightening” or applying the primary coating. Tightening is carried out at low current densities, which ensures the elasticity of the deposited metal. Tightening is carried out in diluted sulfuric acid copper plating electrolytes.
Composition and mode of operation:
| Electrolyte composition and operating mode | Dilute copper plating electrolyte |
| Copper sulfate, g/l | 140-160 |
| Sulfuric acid, g/l | 10-15 |
| Ethyl alcohol, ml/l | 20-30 |
| Temperature, 0C | 18-25 |
| Process time, min | 15-30 |
The model is suspended in a bath under electric current. It is necessary to ensure that when hanging the model there are no air bubbles left in the recesses, otherwise there will be uncovered areas there. After deposition of the primary layer, the model is transferred to a bath for deposition of the working layer.
Working layers are formed, as a rule, from copper, by building up thick layers of nickel or iron. Electrolytes and operating modes are presented below.
Composition and mode of operation:
| Electrolyte composition (g/l) and operating mode | Electrolyte No. 1 | Electrolyte No. 2 | Electrolyte No. 3 | Electrolyte No. 4 |
| Nickel sulfate | 170 | 240 | 140-160 | 360 |
| Nickel chloride | — | 45 | — | — |
| Boric acid | — | 30 | 20-30 | 30 |
| Sodium chloride | 40 | — | — | 40 |
| Sodium acetate | 50 | — | — | — |
| Acetic acid, 80% | 1 | — | — | — |
| Magnesium sulfate | — | — | 25-30 | — |
| Sodium sulfate | — | — | 180-200 | — |
| Potassium chloride | — | — | 5-10 | — |
| Sodium fluoride | — | — | — | 15 |
| Cathode current density, A/dm2 | 4-8 | 5-10 | 0,5-0,8 | 1,5 |
| Temperature, 0C | 70-72 | 50-60 | 36-38 | 40 |
| pH | — | — | 5.6-5.8 | 5.6 |
Copper plating electrolytes for depositing thick layers of copper.
Composition and mode of operation:
| Electrolyte composition (g/l) and operating mode | Electrolyte No. 1 | Electrolyte No. 2 | Electrolyte No. 3 |
| Copper sulfamic acid | 240-260 | 200 | 200 |
| Sulfuric acid | 60-70 | 50 | 30 |
| Anthracene sulfonated | 0,2 | — | — |
| Temperature, 0C | 37-39 | 25-38 | 18-20 |
| Cathode current density, A/dm2 | 4-10 | 2-5 | 1-3 |
| Mixing | + | + | — |
| Filtration | Periodic | Periodic | Periodic |
Electrolytes for depositing thick layers of iron.
To apply thick layers of iron, sulfate and chloride electrolytes are used.
Ferrous sulfuric acid electrolytes.
Composition and mode of operation:
| Electrolyte composition (g/l) and operating mode | Electrolyte No. 1 | Electrolyte No. 2 | Electrolyte No. 3 | Electrolyte No. 4 |
| Iron sulfate | 180-200 | 400 | 350 | 120 |
| Magnesium sulfate | 40 | — | 250 | 20-25 |
| Sodium bicarbonate | 25-30 | — | — | 5-10 |
| Sodium chloride | — | 200 | — | — |
| Cathode current density, A/dm2 | 0,1-0,15 | 10-20 | 10-20 | 3-4 |
| Temperature, 0C | 18-20 | 90-100 | 102 | 75-80 |
Iron chloride electrolytes.
Composition and mode of operation:
| Electrolyte composition (g/l) and operating mode | Electrolyte No. 1 | Electrolyte No. 2 | Electrolyte No. 3 | Electrolyte No. 4 |
| Iron chloride | 450 | 500 | 500 | 700-800 |
| Calcium chloride | 500 | 150 | — | — |
| Sodium chloride | — | — | 950 | 9 |
| Hydrochloric acid | 0,2-0,5 | 3-4 | 2-3 | 3-4 |
| Cathode current density, A/dm2 | 10-20 | 20 | 10-25 | 10-20 |
| Temperature, 0C | 90-100 | 106 | 95-100 | 100-105 |
In decorative galvanoplasty, ironing processes are almost never used, because this is more the prerogative of industrial production, in the manufacture of matrices or molds. In decorative electroplating, copper plating electrolytes and less often nickel plating electrolytes are most often used, followed by applying a thin layer of silver or gold to a copper or nickel model of a product, or some other method of giving the model or product a marketable appearance.
Electroplating is a very delicate process and requires constant control over the product. The processes of galvanoplastic deposition of thick layers can be quite long in time, depending on the required thickness of the deposited layer, and can last from several hours to several weeks.
Types of basic galvanic coatings
A popular metal galvanization is copper plating. They are attracted by the low cost of the starting ingredients and the speed of creating the layer. The electrolyte is created on the basis of copper sulfate. The created layer has good electrical conductivity. It is easy to purchase an electrode made of this metal at a reasonable price.
Coatings are also used:
- gold;
- silver;
- chrome;
- nickel;
- zinc;
- tin.
Multilayer combinations are used to obtain special technical and aesthetic parameters. The following sections will show how the combined use of several metals improves the appearance and other characteristics of the protective and decorative layer.
Important! The compatibility of individual materials must be taken into account. The copper-aluminum galvanic couple is not used. This combination activates the process of electrochemical corrosion. The galvanic couple in this case forms a kind of current source. The magnitude of the EMF of such a “battery” determines the speed of destructive processes.
Special mention should be made of the features of brass plating. Unlike other processes, an electrolyte is used here, which simultaneously contains two main components: zinc and copper. It is from these that the alloy brass is created. In this example, using electrochemical processing, a thin layer is applied to a metal workpiece.
Galvanostegy
Electrotype
Tea and coffee service from the Christofl manufactory, 1875, one of the first uses of galvanoplastic silvering and gilding
Main article: Electroplating
See also: Counterfeiting § Electroplating
Galvanoplasty is one of the branches of electroplating. Shaping from a non-ferrous metal by deposition from a solution (melt) under the influence of an electric current on a matrix. It is used to produce metal copies of objects using electrolysis methods. This term can also be used as a name for metal objects obtained by electroplating. The thickness of metal deposits applied during electroplating is 0.25-2 mm.
Electroplating is most widespread in the manufacture of exact artistic copies of small sculptures and jewelry; in technology - in the production of gramophone records, printing rollers, metal products with micron parameters.
Despite the emergence of new technologies, for example: three-dimensional scanning and three-dimensional printing, casting in elastic molds and lost wax models, etc. Electroplating continues to be the most popular method for producing precise metal copies of small art objects and some other types of products.
Galvanostegy
Electroplating is the electrolytic deposition of a thin layer of metal on the surface of a metal object or part.
Depending on the requirements for the performance characteristics of parts, coatings are distinguished:
- protective (to protect the coated metal from corrosion);
- protective and decorative (to protect the coated metal from corrosion and give its surface a decorative appearance);
- decorative (to give the surface of the metal being coated a decorative appearance);
- special (to give the surface of the metal being coated certain properties, for example: dielectric, electrically conductive, wear-resistant, extreme pressure, solderable, to increase adhesion when rubberizing steel products, etc.);
The same coatings, depending on their area of application, can be classified as protective, protective-decorative, or special[4].
The resulting coatings—sediments—must be dense and fine-grained in structure. To achieve a fine-grained structure of deposits, it is necessary to select the appropriate electrolyte composition, temperature conditions and current density. The choice of coating method depends on the purpose and operating conditions of the product.
Features of galvanic silvering and gilding
Below are technologies that have relatively high consumables costs. For silvering, a solution is created based on well-purified distilled water. Add soda ash, potassium ferric cyanide and the main ingredient - silver chloride.
They maintain a relatively low temperature (from +18°C to +22°C) of the working environment. Current calculations are performed taking into account the density per unit area of the electrode. In this case, 0.1-0.12 A per dm2 is enough. An anode made of graphite is suitable. Its size should be larger than the product being processed.
To apply metal to a dielectric, it is necessary to create an intermediate conductive layer
This electrolyte is created from an aqueous solution of gold mixed with acid. Careful preparation of the workpiece is recommended. To improve adhesion, in addition to thorough cleaning (degreasing), immersion in mercury nitrate is used. To reduce the consumption of ingredients and prevent defects, copper plating is first applied.
Important! To safely use aggressive chemical compounds, intensive ventilation or work operations must be performed outdoors.
Process Features
To obtain a high-quality coating, you need to select the correct current and voltage. If the current is too low, the metal is deposited for too long. If the standard parameters for current and voltage are exceeded, the metal is deposited in flakes. Another point is the purchase of liquid for electrolyte. It is easier to use a car battery solution, but specialized chemicals, such as sulfuric acid, are difficult to obtain for the average person. Most often, this processing method involves copper plating of products. But you can silver or gild the workpiece if there is precious metal.
Gilding with gold leaf leaves looks beautiful, but its cost is much higher than that of gold-plated products in retail sale. The larger the part, the larger the electrode plate and supplied current are required. Therefore, in everyday life, large things are not subjected to galvanoplasty.
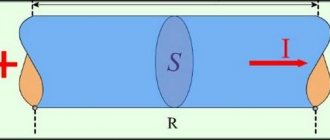
The electroforming process begins with the assembly of the apparatus. The plus from the current source is supplied to the plate, and the minus is supplied to the product. To prevent the wires from starting to react during galvanization, the place where they connect to the plate is covered with plasticine. The area with a positive charge must be larger than the area of the workpiece, preferably at least twice as large. To set the optimal current on the device, use a simple formula. The plate area is multiplied by the current density. Usually they take a density value of 1-2 A per square decimeter.
After completing the calculations, processing begins. The degreased workpiece is attached to the negative contact using glue and copper wire. If the material is not conductive, the product must be treated with graphite spray. If the future decoration imitates jewelry, you need to seal all the stones and glass with plasticine. This material will not allow the color of the stone to change. It is advisable to use glass or stones that are resistant to aggressive environments to create jewelry.
The layer obtained within two hours differs from the layer produced in a day of greater thickness and strength. It is important to consider that the bath with electrolyte and products must remain motionless for many hours for a high-quality result. The finished product does not appear to be iron; it will sparkle with a pinkish copper sheen. This result indicates that the process was successful.
Features of galvanization with various metals at home
Below are the nuances that should be taken into account when reproducing individual technologies.
Nickel plating of metal products
For this process, an increase in temperature (from +24°C to +26°C) and galvanic current up to 1.2 A per dm2 is used, compared to the silvering presented above. The pH value is carefully monitored. The recommended pH range is from 3 to 6. A durable layer will have time to form in 30-40 minutes.
Copper plating without immersion
The steel product is fixed in a holder and connected to a direct current source (minus). A brush made of stranded copper wire is dipped in electrolyte. This instrument is connected to the positive. It is driven over the treated part of the surface.
Electrochemical galvanizing
The electrolyte is created from the following ingredients:
- distilled water – 2 liters;
- ammonium sulfate – 100 g;
- zinc sulfate – 400 g;
- sodium acetic acid – 30 g.
Treatment lasting 30-40 minutes will create a durable layer that well protects parts from corrosion. This method is cheaper than using similar stainless steel parts.
Chrome plating of metal products
For reliability, this layer is fixed on a technological nickel substrate. This solution does not form a galvanic couple. Increasing the temperature increases the shine of the decorative coating. Durable coatings are obtained at a current density of more than 90 A per dm2, which is difficult to achieve at home.
Equipment and homemade devices
Equipment for electroplating is no different from equipment used for electroforming. Any glass can be used as a galvanic bath of such a size that the object to be coated with metal can be placed freely in it and not be too close to the anode plates.
Rice. 1. Galvanic bath in a quadrangular jar.
It is most convenient to use quadrangular glass jars (Fig. 1).
Transverse crossbars are made from thick copper wire or tubes, two of which (a) are used for hanging nickel or copper plates - anodes, and the third (b) - for nickel-plated or copper-plated objects.
Rice. 2. Galvanic bath in a round jar.
In a round jar, the anode plate has to be bent into a cylinder (c) (Fig. 2).
The objects to be coated are suspended on copper wires. There should be two anode plates. It is important that the objects to be coated face the anodes with their largest areas and are approximately in parallel planes with them.
The crossbars from which the anodes and coated objects are suspended must be equipped with terminals for convenience and reliability of connection (see Fig. 3). The wires that attach the anode to the crossbar must be above the level of the Electrolyte, especially if they are made of another metal.
The anode plates are connected in parallel to each other and must be connected to the “plus” terminal of the battery or rectifier current source).
Anodes must be thoroughly cleaned of oxides, dirt and degrease, just like objects intended to be coated with metal.
An important condition for successful nickel and copper plating is cleanliness. If slight turbidity appears in the electrolyte or a precipitate has formed, the electrolyte must be filtered.
In Fig. Figure 3 shows the connection diagram for the galvanic bath. As a source, you can use a car battery or a rectifier (voltage 6-12 V), powered by an alternating current network with a voltage of 127-220 V. A voltmeter and an ammeter must be connected to the circuit. If the surface of the object to be coated is less than 2 dm^2, you can use a 500 mA milliammeter.
The resistance of the rheostat should be about 8-10 Ohms so that the current can be changed within fractions of an ampere.
When assembling the electrical circuit of the bathtub, it is very important not to confuse the poles of the battery or rectifier, since the anode plates must be connected to the positive pole, and the part (object) to the negative. If turned on incorrectly, the metal of the part or object will “dissolve”, which will lead to damage to the electrolyte.
An even, dense coating of an object with nickel or copper depends on the magnitude of the electric current, which does not exceed a known limit and depends on the surface area of the object.
For example, if the standard current density is 0.5 A per 1 dm^2 and the object has a total surface of about 0.5 dm^2, then the current should not exceed 0.5 X 0.5 = 0.25 A. At higher currents nickel or copper will be deposited as a dark, weak layer that comes off easily. If the object has pointed parts, the current density should be reduced by 2-3 times.
Objects are immersed in a bath under voltage. To do this, they are first suspended on bare copper conductors with a diameter of 0.8-1 mm to a crossbar (copper tube), connected to a source of electric current (the rheostat is turned on to full resistance) and lowered into a bath of electrolyte. Then, by reducing the resistance of the rheostat, the current is brought to normal.
Rice. 3. Scheme of connecting a galvanic bath to an electrical circuit.
During galvanization, the part or object is removed from the bath for a short time two or three times and inspected. If the metal is deposited unevenly, change the position of the object, turning it towards the anode with the side on which the metal layer is thinner.
With the correct nickel plating process, the nickel is deposited in a matte, even, silvery layer throughout. The appearance of dark spots indicates poor degreasing. A thin layer of metal is deposited on a part or object in 20-30 minutes, a thick layer in several hours.
An object taken out of the bath, no matter how well it has been previously polished, has a matte surface. To add shine, it is polished with the finest chalk (tooth powder) using a cloth. You can also polish with crocus, but be very careful not to damage the nickel layer.
Note. Aluminum is widely used in amateur designs. Anodizing can be performed with alternating current 12-24 V. The part (sheet) is polished to a mirror shine, wiped with acetone and chemically degreased in a solution of caustic soda 50 g/l. Degreasing time is 3-5 minutes, solution temperature is 50° C.
AC anodizing is as follows. If a part (sheet) is anodized, then it is the first electrode, and the second can be a processed aluminum billet or sheet.
The contacts of the current leads must be aluminum. The electrolyte is a 20% sulfuric acid solution.
Anodizing conditions are as follows.
- For aluminum and clad duralumin, the current density is 1.5-2 A/dm^2 at a voltage of 12 V. Anodizing time is 25-30 minutes, electrolyte temperature is not higher than 25 ° C.
- For unclad duralumin, the current density is 2-3 A/dm^2 at a voltage of 12-20 V. Anodizing time is 20-25 minutes, electrolyte temperature is about 25 ° C.
Galvanic coatings GOST
Galvanization is suitable for solving various problems; what it is from the point of view of professionals can be clarified in specialized standards. The necessary information is given in official standards.
Table of thematic GOSTs
| Document Number | Subjects, features |
| 9.309.-86 | Creation of uniform coatings at an average current density of no more than 5A per dm2. |
| 9.308-85 | Test technologies |
| 12.3.008-75 | Safety regulations |
| 9.005-72 | Acceptable combinations of metals that do not form a galvanic cell |
| 9.313-89 | Creation of coatings on products made of polymer materials |
| 9.908-85 | Determination of corrosion resistance for selection of galvanic isolation unit |
| 12.1.007-75 | Classification of harmful substances |
| ISO 4042 | Creation of electroplating coatings on fasteners |
| 2789-73 | Surface roughness |
Industrial galvanoplasty, Vansovskaya K.M., Volyanyuk G.A., djvu
Practical advice for a galvanizer, 1-105 Lobanov S.A., djvu
Practical advice to a galvanizer, 106-245 Lobanov S.A., Djvu
Electroplating for craftsmen, Vilbiris S., djvu
Silvering, gilding, palladizing and rhodium plating, Burkat G.K., djvu
Electrochemical deposition of noble and rare metals Yampolsky A.M., djvu
Sputnik galvanika, Zaltsman L.G., Chernaya S.M., djvu
Handbook on electroplating in mechanical engineering. Melnikov P.S. djvu
How to make a copy of a coin, how to clean coins from oxides, a simple method of applying gold coating to a coin - from the old magazine “Model Designer” - Watch
Degreasing, etching and polishing of metals, S.Ya.Grilikhes, djvu
Electroplating in industry, Treasurer V.Ya., djvu
Analytical chemistry of gold, A.I.Busev, V.M.Ivanov, djvu
General Chemistry Read online by N.L. Glinka This textbook is intended for students of non-chemical specialties in higher educational institutions and is an excellent tool for those who independently study the basics of CHEMISTRY and for students of chemical technical schools and senior high school students. There is a wealth of information on all kinds of physical and chemical processes in an accessible form. Read online. You can download any of the 731 pages. Here you can watch the tutorial with all the pages downloaded at once.
If anyone wants to test and strengthen their knowledge of general chemistry, scans of the book “Problems and exercises in general chemistry” are at your disposal. 2005
I am now posting here the twenty-eighth edition of this wonderful textbook by N.L. Glinka on general chemistry, published in 2000.
Maybe someone will find the workbook “Learn to solve problems in chemistry” useful. 1986 by N.N. Magdesieva, N.E. Kuzmenko
Entertaining electroplating technology Djvu Odnoralov N.V. The manual outlines methods and techniques for performing all kinds of galvanoplastic coatings: silver plating, nickel plating, chrome plating, etc. Much attention is paid to the production of simple clichés for the school of decorative metal products. The technology and recipe offered in the book are simple and accessible. The purpose of the book is to broaden the horizons of students and instill in them the necessary practical skills.
Refining of gold, silver and platinum group metals djvu Textbook for universities 1945 This book is intended to serve as a textbook for students of higher technical educational institutions of non-ferrous metallurgy specializing in the field of metallurgy of precious metals
Reference Guide to Electroplating - 1969 pdf Liner V.I. Materials reflecting the current state of electroplating technology in various countries of the world are presented. Theoretical, chemical and electrical engineering justifications for electroplating processes are presented, information is provided regarding the choice of material to be electroplated equipment, various surface pre-treatments, methods for applying various protective coatings, adjusting the electrolyte and testing the applied coatings.
Galvanic coatings, 2000 pdf Sidneev Yu.G. The book contains a large amount of practical material on electroplating. Various methods of applying metal coatings using galvanic and chemical methods in small enterprises and workshops are described. The types of electrolytes, optimal deposition modes and features of their operation are described in detail. Anodizing and painting of aluminum alloys, as well as painting of steels, copper, silver and zinc alloys are described. The material in the book is presented in such a way that it can be used by a wide range of readers familiar only with the basics of chemistry and physics. A separate chapter is devoted to galvanoplasty. It outlines issues related to the manufacture of forms for electroplating, the preparation of the surfaces of these forms and other technological operations. The book is intended for entrepreneurs engaged in production activities on a small scale, as well as for those people who like to do everything with their own hands.
Galvanic and chemical coatings with precious and rare metals. Djvu The collection was prepared by the section Protective and decorative coatings of the Moscow House of Scientific and Technical Propaganda named after F.E. Dzerzhinsky, in conjunction with the section of metallurgy and processing of the Moscow Board of the Scientific and Technical Organization of Non-ferrous Metallurgy based on materials from the seminar GALVANIC AND CHEMICAL COATINGS WITH PRECIOUS AND RARE METALS. The purpose of the seminar is to exchange experience between specialists from industrial enterprises, research, development and design organizations of various industries on the issues of applying galvanic coatings with precious and rare metals and alloys based on them.
Galvanoplasty djvu Sadakov G. A. 1987 The book is devoted to the technology and use of galvanoplasty in mechanical engineering, electrical engineering and other industries. Information is provided on materials (metals, non-metals) for the manufacture of molds, basic designs of molds, technological features of their use, methods of modifying the surface of the mold (cleaning, applying separating or electrically conductive layers). The features of the deposition of thick layers of nickel, cobalt, copper and alloys based on them, their structure and properties are considered. Methods of technical control of some basic characteristics and parameters of forms, copies, products and electrolytes are reflected. Information is provided on the technological equipment of workshops and areas of galvanoplasty, examples of the industrial application of galvanoplasty. For engineering and technical workers of machine-building plants, special design and technology bureaus, and research institutes.
Electroplating in industry djvu Treasurer B.Ya. Recently, galvanoplasty has been gaining an increasing place in technology and industry. If previously it was used only for the manufacture of sculptures, electrotypes and matrices of gramophone records, now it is increasingly used for the manufacture of parts of complex shapes, matrices for pressing plastics, and stamps for embossing. The most important applications of electroforming include the manufacture of waveguides for radar, printed radio circuits, thin sieves with 10,000 holes per square centimeter, electroforming installation, etc. In modern technology, products or parts that are uneconomical, difficult or impossible to produce by casting are prepared by electroforming. forging or machining. In addition, electroplating techniques are used to apply a metal layer to non-conductors, such as plastics, porcelain, wood, plaster, rubber, wax, quartz, etc. This allows metal to be added to lace, fabric, hair, leaves, flowers, fruits and even small animals. Coating non-conductors with metal, in addition to saving the latter and simplifying the design, makes it possible to impart new physical and mechanical properties to products, which are successfully used to solve design problems; for example, in this way they combine in one product dielectric properties with electrical conductivity, thermal conductivity with thermal insulation, increased mechanical strength with low weight of products, etc.
Theory and practice of jewelry. E. Brepol. Read online
Preparation of pure chemical substances Read online Karyakin Yu.V. 'Pure chemicals' Guide to the preparation of pure chemicals, including organic ones (hydrazine salts, etc.) - acids, metal salts, alum, including methods of purification from impurities.
Galvanic coatings of dielectrics Read online Data are provided on the implementation of all operations of technological processes for obtaining coatings: methods for preparing solutions and electrolytes, their compositions, processing modes in them, adjustments and operation, main problems that are possible during coating processes, their causes and methods of elimination. The classification, properties and scope of application of galvanic coatings on dielectrics are described. For NTP, craftsmen and qualified workers in electroplating workshops.
Galvanoplasty, galvanostegy, patination
Galvanoplasty is called copying technology. The essence of the processes does not differ from the above descriptions. However, adhesion is reduced to facilitate separation of the finished product from the workpiece.
Electroplating is an improvement in the mechanical parameters of the combined layer. Chrome, for example, prevents damage to steel products due to its high strength.
Patination is used to change the decorative properties of the surface. In particular, they create an artificially aged appearance.
Arrows mark areas created using the “rainbow” patination technology
Application of galvanoplastics in industry.
The classification of the use of galvanoplastics in industry mainly comes down to a consideration of galvanoplastics by industry or manufacturing technology; tools and equipment are also distinguished separately. Next, I will present examples of the application of the electroforming process in the production of specific parts and products that are most interesting in my opinion.
3.1 Production of seamless pipes of various profiles and complexity.
The process of manufacturing thin-walled pipes without a seam using the electroplating method was first implemented in Russia. THEM. Fedorovsky produced straight, complex bent pipes with branches of different diameters and different wall thicknesses using the galvanoplastic method. His method was as follows: pipes were made by depositing copper on a cathode, which was a rotating copper or iron rod; the current density in the process ranged from 2 to 6 A/dm2; An agate stone moved along the rod, smoothing and compacting the sediment (the process of removing the pipe from the mold was not described).
Figure 1 – An example of a complex profile pipe made by electroplating.
In practice, pipe production is carried out in many different ways. One of these methods is metal deposition on a cylindrical form horizontally located in the bath. The bases of the cylinder are covered with an insulating mass to prevent copper from depositing on them. The wooden axes of the mold are placed in glass bearings, and a rotational movement is imparted to the cylinder. Rotation speed - 40 rpm, current density 1.2 - 1.5 A/dm2. Copper anodes are located at the bottom of the bath. A pipe with a wall thickness of 3.2 mm takes 144 hours to grow. Upon completion of the process, the pipe, along with the mold, enters the flaring machine and is then removed. Venturi tubes for measuring fluid flow are prepared as follows.
Figure 2 - Venturi pipes. At the top are the forms, at the bottom is an extended pipe with soldered flanges and bends
The first step is to make an aluminum alloy mold. They are prepared mechanically or by injection molding. The molds are carefully ground, polished, glossed, after which they are degreased in an organic solvent, then in an alkaline solution, and then washed. Before hanging the forms in the bath, they must be decapitated in a mixture of nitric and hydrofluoric acids. This step is necessary to remove the oxide layer from the surface of the aluminum, and this in turn improves adhesion and increases the tightening speed.
Tightening of the aluminum mold must be done in a bath of low acid concentration and high current density. When the form is completely tightened, it is transferred to the extension bath. At the end of the extension, the form is dissolved in concentrated caustic soda or hydrochloric acid. The inner surface of the tube is coated with a thin layer of silver.
3.2 Production of waveguides.
The technological process for manufacturing waveguide elements consists of the following main operations: preparing the surface of the mold (degreasing, applying a separating or “protective” layer), electrodeposition of a thin layer of gold or silver, deposition of a base layer of copper (or nickel) 1.5-2.0 mm thick , extracting the form from the resulting copy.
Figure 3 – Fragment of a waveguide: 1 – electrodeposited metal layer, 2 – molds
If it is necessary to obtain a strong but lightweight structure, then the process of enveloping electrodeposited metal layers with plastic is used. In the production of waveguides, both permanent and composite forms are used. Form materials are selected depending on the situation. So, if the configuration of the waveguide assembly does not allow the mold to be removed without destroying it, then the mold is made from aluminum and its alloys, and sometimes from zinc alloys. Molds from these materials are removed by dissolution. For the manufacture of permanent forms, corrosion-resistant steel is widely used, which makes it possible to easily separate the metal layer without special preparation. But it is not always suitable for making molds, especially small cross-sections, due to its relative softness, and this steel is also inferior in mechanical strength to chromium and tool steels. In the production of waveguide units, steel grades 40Х13, 30Х13, 20Х13 are most often used. To build up basic metal layers in waveguide technology, pyrophosphate and sulfamate copper plating electrolytes, as well as sulfate and sulfamate nickel plating electrolytes, are most often used. In the case of manufacturing channels of complex design, it is recommended to deposit nickel from a citrate electrolyte. This electrolyte has better dissipative ability, but it is less stable in operation compared to sulfate and sulfamate electrolytes; nickel deposition in it occurs at a low rate. When using molds made of structural steel, citrate electrolyte does not cause corrosion (pH= 7-8).
3.3 Obtaining bellows.
Bellows are thin-walled corrugated tubes of various diameters.
Aluminum alloys are used to make molds, since the bellows design does not allow the use of permanent molds. The finished aluminum tube mold is shown in Figure 4.
Figure 4 – Aluminum pipe mold for electrolytic forming of bellows
The mold is cleaned of contaminants and, using a special contact device, after zincate treatment and washing, it is suspended in a galvanic bath. After metal deposition, the mold is etched in a solution of hydrochloric acid and the part is obtained. Most often, deposition is carried out from a nickel sulfamate electrolyte. When deposited onto the mold shown in Figure 5, there is a significant difference in the thickness of the deposit on the depressions and on the protrusions. For this reason, the process is best carried out at low current densities (ik=1.0-1.5 A/dm2). Due to this, a more uniform distribution of nickel deposits across the form is achieved.
Figure 5 – Contact device for electrolytic forming of bellows.
3.4 Cases with a shaped charge.
Shaped charge casings are devices that focus the chemical energy of an explosion to achieve predetermined effects. The main uses are in armor-piercing projectiles and as penetrators in oil drilling.
Figure 6 – Shape and finished sleeves. They are usually made in several ways. For example, metal pressing is the most cost-effective. However, certain special applications require not only a very high degree of precision (especially concentricity), but also chemical properties. The quality of the surface, both inside and outside, is also important. All these parameters can be achieved in the electroforming process. So the molds are made of aluminum. They go through the standard stages of preparation. The most commonly used are acidic copper electrolytes. Removal of the mold is possible mechanically and by treatment in hydrochloric acid.
3.5 Molds and dies.
Galvanoplasty is used as a method for manufacturing form-building parts (inserts) of molds and dies. The manufacturing process of inserts is largely determined by the material of the mold. When using a metal mold, the technological scheme is simpler, but due to the large amount of mechanical processing and fine-tuning of the mold, the cost of the resulting products increases significantly. The process of manufacturing inserts for this case includes the following main stages: preparing the surface of the mold, obtaining a working layer (deposition of nickel and nickel-cobalt alloy), creating a structural layer and fastening the inserts in the matrix. When using non-metallic materials, metallization of the mold surface is necessary. In this case, during mass production, it is advisable to initially produce an intermediate master mold in which the required number of molds necessary for subsequent use in the manufacture of molds can be cast. The master mold is produced using electroforming. Below are all kinds of applications and examples of the use of electroforming.
3.6 Artistic electroforming.
In this case, we consider the technology of making sculptures from composite copies, i.e. replica parts for sculptures are obtained on separate forms - fragments of sculptures. The technology using gypsum molds can be briefly described as follows. Initially, sculptures are made (primary form) from some material (usually clay). After which the sculpture is divided into individual elements, and the boundaries between them are drawn, which determines the quality of the sculpture. Plaster copies (secondary form) of individual elements are removed from the primary form, from which metal copies are made using an electrochemical method. The prepared plaster molds are thoroughly dried and impregnated. The impregnation can be a wax composition. After impregnation, the mold is dried and an electrically conductive graphite layer is applied to it. When electrochemically growing a copy from a mold in a galvanic copper plating bath, acidic sulfuric acid electrolytes are used; sometimes stirring of the electrolyte is required (for example, purified air). The copies obtained separately are mounted one next to the other, thus completing the process of creating a galvanoplastic sculpture. Installation work includes: making frames for three-dimensional sculptures, soldering individual copies together, cleaning seams after joining parts, etc.
Examples of statues created by electroplating are shown in Figure 7.
Figure 7 - Examples of statues created by artistic electroplating.
3.7 Other applications of electroforming.
3.7.1 Thin-layer products.
Nickel Foil: Fire Retardant Blankets, Seamless Tapes, High Temperature Gaskets (with Graphite), Photovoltaic Cell Substrate, Solar Absorbers. Nickel mesh: electron microscope gratings, sugar centrifuge sieves, electric razor, battery screens, screen printing, platinum sieves for fuel cells, spray paint masks.
Copper foil: printed circuit boards.
Iron foil: packaging.
3.7.2 Tubular products.
Nickel: patterned textile printing cylinders, capillary columns for gas/liquid chromatography, nickel-plated diamond-coated drills, ultra-precision components for X-ray telescopes, nozzles for inkjet printers and uranium enrichment, bellows, waveguides, Venturi tubes. Gold: tubes for jewelry.
3.7.3 Duplicate plates.
Analogue and digital audiovisual recordings, Fresnel lenses, holograms, printing and embossing plates.
3.7.4 Forms and equipment.
Nickel: low pressure/low temperature molds for pressing, injection molding of plastic, rubber, glass, zinc. Electroformed tools, including press tools, casting molds, diamond cutting belts, abrasive sheets and copper spark erosion tools. Nickel/cobalt, nickel/manganese or nickel-phosphorus/silicon carbide: Harder forms with higher temperature resistance.
Copper: forms that require good thermal conductivity.
3.7.5 Optics.
Videodiscs and holographic stamps; X-ray telescopes; range of metal optics, including complex aspherical reflectors.
Galvanic pair of electrodes
Galvanic cells are conductors made of different materials. The second prerequisite for this term is the connection of the circuit to ensure electrical contact and the generation of electromotive force between the contacts. Immersing such parts in a solution with clearly expressed alkaline (acidic) characteristics activates corrosion. To prevent rapid destruction, in addition to the copper-aluminum pair, the following combinations are not recommended:
- titanium-aluminium;
- tin-silver;
- lead-platinum;
- nickel-magnesium alloy, etc.
Gilding and silvering
Coating a metal with a layer of silver or gold is not only a galvanoplastic processing method, in which an exact copy is obtained from the surface of the processed product, but also a technology that makes it possible to create a protective and conductive layer on the part. To apply silver to a ferrous metal part, it must first be coated with nickel.
The electrolyte for silver plating includes potassium ferric cyanide, sodium carbonate and distilled water. The operating temperature of such a solution should not exceed 20°. Graphite plates are used as anodes when performing silvering by electroplating.
For silvering, parts are dipped into an electrolyte containing a metal salt, for example, silver nitrate.
Electroplating at home is also possible, during which the surface of the product is formed using a layer of gold. In addition, using this technology, simple gilding of a part can be performed. In this case, an aqueous solution of gold with potassium bluehydride is used for electroplating. You can work with such an electrolytic solution only in rooms with a good ventilation system.
Many home craftsmen are wondering how to make the gilding process safer for human health. To solve this problem, the poisonous acid can be replaced with potassium ferrous sulfide, which is also called blood salt. Before gilding at home, the product is thoroughly cleaned and plated with copper if it is made of steel, lead, tin or zinc. To improve the adhesion of the gold layer to the surface being treated, the product is dipped into a solution of mercury nitrate before treatment.
Silvering with heating of the workpiece
Cooking at home:
- Mix 100 g of argentum chloride with water;
- add 600 g of table salt and cream of tartar;
- Bring to a thick, homogeneous mass.
The resulting paste should be stored in a dark glass container.
Principle of use:
- dilute the mixture (3 tbsp) with boiling water (5 l) in a copper container;
- place the item in the grill for 15-20 minutes.
This method of electroplating with silver will not add brightness. To get shine, you should use another recipe:
- 100 g acetic acid;
- 300 g sulfur salt;
- 4.5 liters of water.
Preparation:
- boil water to 75...80 °C;
- mix the ingredients and add to the liquid;
- put an item;
- cook for 15 minutes.
Immersion method of silvering
Equipment you will need:
- graphite rod;
- power unit;
- electrolyte for electroplating with silver.
Liquid composition:
- 1 liter of distilled water;
- 15 g yellow blood salt;
- 25 g soda ash;
- 15 g silver chloride.
Preparation:
- to boil water;
- mix the ingredients;
- cook for 2 hours.
Store the solution in a dark place and shake before use.
Is it possible to plate silver with gold?
Gold plating is electroplating. To gild silver items, certain equipment is required. In addition, the labor-intensive process is an expensive service, hardly available at home: when covering the material with aurum, high-grade metal (750-999 samples) is used. It is best to go to a jewelry workshop to have the item processed by a professional.
Safety precautions during work
Be sure to check the compliance of the power supply network with high power consumption. If necessary, use a separate line, which is connected in the electrical panel to a separate circuit breaker. The DC source is grounded. Only serviceable equipment is used.
To carry out work safely, it is better to use a garage, other technical room, or an outdoor area. Additionally, standard personal protective equipment is used:
- latex gloves;
- respirators, gauze bandages;
- transparent masks, glasses;
- clothes with long sleeves.
History of development and advantages of electroplating
The basis for the technology is the publication of the famous Italian L. Galvani “On the Forces of Electricity” (1779). The first working method was created by B. Jacobi in 1838. Even then, good practical results were obtained. Centuries of process improvement now provide the following advantages:
- high processing accuracy even for complex-shaped products;
- the ability to control the thickness of one or several layers;
- high-quality uniform coating structure;
- good grip on different surfaces;
- excellent aesthetic characteristics.
The listed advantages can be used if the technological rules are followed correctly. Preparation is essential. Sufficient attention should be paid to the selection of equipment.
Electrotype
Fig.8. Mold (a) and model (b)
Having enclosed the counter-relief in a steel ring (Fig. 8), lead or babbitt is poured into it, which is soldered to the tinned side of the counter-relief. The ring is easy to cut from a pipe of the appropriate diameter. To avoid spreading of lead, the counter-relief is pressed into sand, previously poured onto asbestos. The outer edges of the ring should also be covered with sand. In the mold obtained in this way, you can press products from plastic mass - organic glass, after softening it over an electric stove or in boiling water to the state of rubber. For pressing, softened organic glass is placed on the resulting mold, on top of which thick sponge rubber is placed, and then a steel tile 4-5 mm thick.
The prepared mold is placed on a press and the mass of organic glass is compressed for 3-5 minutes until almost completely hardened. After pressing, the mold along with the model is immersed in water for final cooling and the model is removed from the finished mold by lightly tapping. To extrude models of small sizes (5-6 cm), a stationery screw press or a hydraulic press is enough, the molten composition is then graphitized, a conductor and a load are attached.
PRODUCTION OF PRESS FORMS
Requirements for forms:
Materials used for the manufacture of molds for galvanoplastic reproduction of sculpture must meet the following basic requirements:
— easy to separate from molded objects and give accurate prints; — have minimal shrinkage during setting and hardening, and do not deform when cooling or drying; - not be hygroscopic, be harmless to the electrolyte, not pollute it and not be destroyed by prolonged exposure to the electrolyte; - easy to bond with the applied electrically conductive layer.
Plaster molds
Making plaster molds from round sculptures. Obtaining three-dimensional forms from gypsum for the purposes of electroplating when reproducing artistic sculpture does not differ in special techniques from conventional molding. True, due to the fact that gypsum forms are hygroscopic, they have to be subjected to additional impregnation in ozokerite, wax or paraffin. As noted above, rough forms usually consist of two, sometimes three shell pieces. The rough form of two shells is the easiest for electroforming; With this form, it is convenient to both apply an electrically conductive layer to the inner surface (cavity) of the mold and carry out the electrolysis process. Metal is added to each sink separately. To mount the sculpture, the connecting seams of the shell are prepared and then soldered. To obtain a reproduction that does not require adjustment and soldering of two parts, resort to the following technique. Pre-prepared shell-forms, impregnated with a wax composition and coated with an electrically conductive layer, are precisely connected to one another and tied together with an insulated wire. Then, using plasticine that does not contain filler or pigment, the seam at the junction of the shells is sealed from the inside. Yellow ozokerite is most suitable for sealing seams: it has high ductility and viscosity.
The same technique can be used when working with piece molds, but there it is less convenient due to the bulkiness of the piece molds, the presence of a casing and a large number of seams formed by individual pieces of the mold, especially since the seams still remain noticeable on the metal reproduction. The use of conventional rough forms with metal extension into each sink separately is the simplest and most technically convenient, but requires adjustment of the sink. The use of pre-connected shells of a rough form with seam development does not require mounting work to connect parts of the finished metal reproduction, but this method complicates the electrolysis process: it makes it difficult to uniformly build up metal in the deepest places of the form. The use of conventional rough forms is advisable mainly when making large sculptures. Individual parts can be produced in lump molds, and then the finished parts can be soldered together.
Making plaster molds from bas-reliefs. When removing molds from bas-reliefs, medals and other artistic products that have a relief without undercuts (“locks”), the molds are made by filling the models with plaster. To do this, pour gypsum into water and stir it, obtaining a creamy mass. It is first recommended to apply plaster onto the relief with a brush, so that in the resulting form there are no places not filled with plaster and no traces of air bubbles. After applying a thin layer of plaster to the surface of the model to be copied, fill it with diluted plaster. In this case, the work should be done “at pace”, since the diluted plaster hardens quickly. To remove plaster molds from plaster models, the latter are pre-lubricated with a solution of paraffin in kerosene, which prevents the plaster model from merging with the plaster mold being manufactured. Such forms are easy to remove not only from metal, wooden and plastic models, but also from plasticine and clay. In this case, however, the models are usually destroyed when the mold is removed. To avoid spreading of the poured plaster, a shell (rim) made of cardboard, Whatman paper or foil is installed around the model. When the gypsum has cooled and completely hardened, remove the shell, trim the edges of the mold with a knife and make a hole in the edge of the mold for the electrical wire necessary for hanging and contacting the negative pole of the current source.
Impregnation and insulation of gypsum forms.
Impregnation of gypsum forms to eliminate hygroscopicity is one of the most important operations. Before impregnation, gypsum forms are well dried at a uniform temperature increase to 50-60°C. The temperature should be increased especially slowly when drying very damp molds that have just been removed to avoid deformation and cracks. Forms consisting of several pieces are dried assembled with the pieces pressed tightly against one another (to eliminate their deformation). In the drying cabinet, the molds are placed on a perforated rack (with holes) with the open part facing up, so that the molds are evenly heated from below, and the moisture escapes to the top. A well-dried form has a completely white color and, when tapped, produces the specific sound of dry plaster. This form quickly absorbs impregnation and does not crack. Plaster molds are impregnated with molten wax compounds with a melting point from 50 to 125 ° C; compositions having a low melting point are preferred. The forms immersed in the impregnating composition are heated, and the air in the pores of the forms is displaced. The depth of impregnation depends on the soaking time of the gypsum forms in the composition; a sufficient thickness is 2-5 mm.
The higher the temperature of the impregnating composition (and it can be significantly higher than its melting point), the less the molds can withstand it. The thicker the walls of the mold, the longer it takes to prepare and soak. Massive thick-walled forms should be impregnated with not too overheated compounds to avoid the destruction of gypsum at elevated temperatures; The most suitable compositions are those with a melting point of 60-80°C. These are, for example, compositions based on petrolatum, ceresin, ozokerite and stearin bases with rosin additives. Impregnation is carried out with exposure in the appropriate composition for 2-2.5 hours. Treatment with a composition with a higher melting point or a superheated composition is used only for small thin-walled forms with short-term exposure (no more than 10-20 minutes). The melting point of various substances used for impregnation can be found in the relevant reference books. Ceresin varieties with a high melting point are used to impregnate molds without mixing with other materials. However, it is most advisable to carry out impregnation in ozokerite compositions, formulated so that they have all the qualities necessary for impregnating compositions.
The main requirements for impregnating compositions include:
— low melting point; — good penetration into the pores of molds; — the property of not softening during normal heating of the electrolyte; — fairly good adhesion to electrically conductive compounds applied to the mold; — the ability not to grease electrically conductive compounds (to avoid an increase in ohmic resistance); — good wettability with electrolyte; — no interaction with electrolyte; - high flash point.
Typically, impregnating compositions consisting of two or three components are used to impregnate gypsum forms. The compositions of some of them are given below, % (by weight):
• 1st composition (melt temperature 85°C)
— Ozokerite 70 — Wax montan composition 30
• 2nd composition (melt temperature 64.5°C)
— Ozokerite 70 — Wax montan composition 15 — Rosin 15
• 3rd composition (melt temperature 75°C)
— Ozokerite 85 — Rosin 5 — Stearin 10
• 4th composition (melt temperature 82°C)
— Wax montan composition 80 — Petrolatum 20
Forms can also be impregnated with clean but hard ozokerite.
Wax molds
Recipes for wax compositions. Wax compositions are convenient for direct pouring onto metal models, as well as onto plaster models moistened with water to prevent sticking, or other models that can withstand the high temperatures of wax compositions (70-80°C). Due to the fact that the properties of the substances included in these recipes are very unstable, recipes should not be copied blindly, but adapted to specific conditions, taking into account the characteristics of the individual components of wax compositions. Paraffin reduces the softness of beeswax, but increases shrinkage; spermaceti facilitates mixing of components; the introduction of excess stearin should be avoided, as it reacts with the electrolyte of the copper bath; mineral oil and beef fat soften the composition, but lead to greasing of the conductive layer. Turpentine has a strong softening effect; it does not spoil the conductive layer, but during long-term storage of the wax composition it evaporates from it.
Graphite slightly increases the electrical conductivity of the molds and facilitates the subsequent graphitization process. Settled during the casting of wax molds in the molten mass of poured wax, it concentrates on the working surface of the mold, where it improves the binding of the wax to the graphite applied to the mold during the graphitization process.
For the manufacture of molds, the following compositions are recommended, % (by weight):
• 1st squad
— Rosin 70 — Beeswax 20 — Paraffin 10
• 2nd squad
— Beeswax 30 — Stearin 70
• 3rd squad
— Beeswax 60 — Ozokerite 15 — Rosin 15
It is best to melt wax compositions in a steam bath, worse - in a sand bath, poorly - on a fire (necessarily low) to avoid the wax flashing and burning to the bottom of the vessel. Melting must begin with the most fusible components and gradually introduce higher melting ones; With flammable components, such as turpentine, you should work further from the fire source, and introduce them last. When contaminated, wax should be filtered through gauze, and very thin wax compositions that do not contain graphite should be filtered through silk. Making molds for bas-relief and medal sculpture. Wax molds, the use of which ensures high precision of reproduction, are used mainly for medallion and bas-relief sculptures. The comparative cheapness of wax compositions, their good connection with the applied electrically conductive layer, the ease of manufacturing wax molds, and the increased accuracy of reproductions make such forms the most common in the electroplating technique. Disadvantages: shrinkage of wax compositions, as well as the inability to use wax forms for repeated reproduction.
Pouring the wax mold
To make the molds, the wax composition is melted and poured into a reproducible metal or raw plaster model. When pouring bas-reliefs, medals and other similar sculptures, you will need metal shells in the form of rings, rectangles, etc. with a wall height corresponding to the height of the relief of the sculpture being poured. Thus, to reproduce a medal sculpture, usually round and with low relief, ring shells with a wall height of 10-15 mm are required. To create a form that is sufficiently strong and does not deform during final cooling, the height of the shell walls should be the higher the height of the relief, the larger the area of the bas-relief. In Fig. Figure 9 shows the moment of filling a wax mold using a shell.