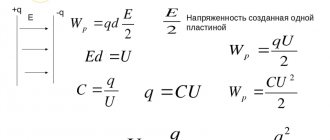The scientific substantiation of many electrical phenomena became possible thanks to Coulomb’s experiments, on the basis of which the scientist introduced the term “point electric charge.” While exploring the nature of electrification, the French physicist, using the torsion balance he invented, discovered the law of interaction of point charges, known to us as Coulomb's law.
Subsequently, this fundamental law helped scientists form an understanding of the structure of atoms and explain the nature of electricity. This contributed to the creation of sources of electric current, without which the current level of scientific and technological progress would not have been achieved.
Story
Thinkers even before our era paid attention to the existence of electric charges. However, they were not able to explain their nature, much less describe their interaction.
Many centuries passed before scientists began to seriously study electrical phenomena, which led them to discoveries in this area. In particular, William Gilbert, back in the 16th century, not understanding the nature of electricity, called bodies that attracted other substances electrified.
In 1729, observing the electrification of various bodies, Charles Dufay came to the conclusion that there were two types of charges, which he called “glass” (since they manifested themselves on a glass rod) and “resin” (arising from the electrification of resins). Later, Benjamin Franklin replaced the terms "glass" and "resin" with the more general terms "positive" and "negative." We still use these terms today.
Despite the fact that these researchers understood the fact of charge distribution, they were unable to explain the nature of the phenomenon. The physicist C. Coulomb came close to understanding elementary particles as charge carriers. The term “point charge” he coined helped the scientist understand the interaction of elementary particles, which led him to the discovery of the law.
Based on his discovery, the physicist could already explain the reason for the interaction of point charged bodies (see Fig. 1).
Rice. 1. Interaction of electrified bodies
The discreteness (indivisibility) of elementary charged particles was proved by Robert Millikan. The scientist confirmed that a charged body contains an integer number of elementary particles. He came to the conclusion that charge divisibility has a limit. The carrier of the elementary charge is the electron.
Figure 2 shows an experiment confirming charge fissibility. Experience shows that division is multiple, which suggests the existence of elementary particles.
Rice. 2. Charge divisibility
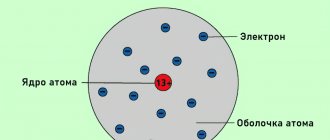
A complete picture emerged after the publication of the visual planetary model of the atom proposed by Rutherford. The model assumes that an atom consists of a nucleus around which electrons revolve. This is a rather simplified model, but it has already explained many electrical processes, including the electrification of bodies.
Rice. 3. Modern interpretation of the planetary model of the atom
Coulomb's law
In 1785, the French physicist Charles Coulomb experimentally established the basic law of electrostatics - the law of interaction of two stationary point charged bodies or particles.
The law of interaction of stationary electric charges - Coulomb's law - is a basic (fundamental) physical law and can only be established experimentally. It does not follow from any other laws of nature.
If we denote the charge modules by | q
1|
and | q
2|, then Coulomb’s law can be written in the following form:
\(~F = k \cdot \dfrac{|q_1| \cdot |q_2|}{r^2}\) , (1)
where k
– proportionality coefficient, the value of which depends on the choice of units of electric charge. In the SI system \(~k = \dfrac{1}{4 \pi \cdot \varepsilon_0} = 9 \cdot 10^9\) N m2/C2, where ε0 is the electrical constant equal to 8.85 10- 12 C2/N m2.
- the force of interaction between two point stationary charged bodies in a vacuum is directly proportional to the product of the charge modules and inversely proportional to the square of the distance between them.
This force is called Coulomb force
.
Coulomb's law in this formulation is valid only for point
charged bodies, because only for them the concept of distance between charges has a certain meaning. There are no point charged bodies in nature. But if the distance between the bodies is many times greater than their size, then neither the shape nor the size of the charged bodies significantly, as experience shows, affects the interaction between them. In this case, the bodies can be considered as point bodies.
It is easy to find that two charged balls suspended on threads either attract each other or repel each other. It follows that the forces of interaction between two stationary point charged bodies are directed along the straight line connecting these bodies. Such forces are called central
. If we denote by \(~\vec F_{12}\) the force acting on the first charge from the second, and by \(~\vec F_{21}\) the force acting on the second charge from the first (Fig. 2 , 3), then, according to Newton’s third law, \(~\vec F_{12} = -\vec F_{21}\) .
- Rice. 2
- Rice. 3
Knowing the law of interaction of point charged bodies, one can calculate the force of interaction of any charged bodies. To do this, bodies must be mentally broken down into such small elements that each of them can be considered a point. By adding geometrically the forces of interaction of all these elements with each other, we can calculate the resulting interaction force.
The discovery of Coulomb's law is the first concrete step in studying the properties of electric charge. The presence of an electric charge in bodies or elementary particles means that they interact with each other according to Coulomb's law. No deviations from the strict implementation of Coulomb's law have currently been detected.
Coulomb's law is also valid for charged balls at any distance between their centers, if the volume or surface charge density of each of them is constant. (Note that, unlike gravitational interaction, electrostatic interaction can lead to attraction and repulsion of bodies.)
see also
- Coulomb's law
What is an electric charge?
This term means that a charged body is capable of creating an electric field. In a broader sense, charge is the amount of electricity - a scalar quantity that is a source of an electromagnetic field and participates in the processes of electromagnetic interactions. Electric charge cannot exist without a carrier.
The elementary carriers of negative charges are electrons. The antipode of an electron is a positron - a stable antiparticle, equal in mass to an electron, but with a “+” sign. There is another stable, positively charged elementary particle - the proton.
Particles charged with fractional parts (quarks) can only exist as part of hadrons, so they are not considered carriers.
The charged protons that make up the nucleus of an atom are tightly bound together by nuclear forces. They cannot escape freely from the nucleus of an atom. Therefore, an ion is considered to be a free carrier of a positive charge - an atom from whose orbit an electron has been removed. The formation of negative ions occurs due to the addition of free electrons to them.
The charge of neutral atoms and molecules is zero, and the number of positive and negative ions in the cells of crystal lattices is compensated. Therefore, bodies under normal conditions are electrostatically neutral. There is no interaction between neutral atoms.
Conductors in an electric field
Conductors are substances in which the ordered movement of electrical charges can occur, i.e., electric current can flow.
Conductors are metals, aqueous solutions of salts, acids, and ionized gases. Conductors contain free electrical charges. In metals, the valence electrons of atoms interacting with each other become free.
If a metal conductor is placed in an electric field, then under its action the free electrons of the conductor will begin to move in the direction opposite to the direction of the field strength. As a result, an excess negative charge will appear on one surface of the conductor, and an excess positive charge on the opposite surface.
These charges create an internal electric field inside the conductor, the intensity vector of which is directed opposite to the external field intensity vector. Under the influence of an external electrostatic field, conduction electrons in a metal conductor are redistributed so that the resulting field strength at any point inside the conductor is zero. Electric charges are located on the surface of the conductor.
Important! If there is a cavity inside the conductor, then the voltage in it will be zero regardless of what field is present outside the conductor and how charged the conductor is. The internal cavity in the conductor is shielded (protected) from external electrostatic fields. Electrostatic protection is based on this.
The phenomenon of charge redistribution in an external electrostatic field is called electrostatic induction .
Charges separated by an electrostatic field cancel each other out if the conductor is removed from the field. If such a conductor is cut without taking it out of the field, then its parts will have charges of different signs.
Important! At all points on the surface of the conductor, the voltage vector is directed perpendicular to its surface. The surface of the conductor is equipotential (the potentials of all points on the surface of the conductor are equal).
Properties
It has been established that the stationary charge q is inextricably linked with the electric field, a representative of a special type of matter. The field is the material carrier of interaction between elementary particles. This property of the field manifests itself even in the absence of matter between the interacting bodies.
The electric field acts with a force F on a test charge q′ located at any point in the field.
Vector quantity:
characterizes the action of electricity and is called field strength. Lines whose tangents coincide with the tension vector form tension lines. The density of the tension lines determines the magnitude of the tension.
The electrostatic field strength lines of a point charge represent rays leaving one point (for positive) or entering a point (for negative) (see Fig. 4).
Rice. 4. Field strength lines
The electrostatic interaction of electromagnetic fields can be observed in the behavior of charged balls. If an ebonite or glass rod is electrified by friction and brought closer to tiny elderberry balls, then we will see how, as a result of force interactions, the particles repel (if they are of the same sign) or attract (if they are of different signs).
The saturation of free charge carriers of different substances is not the same. Most free electrons are found in metals. Since charged electrons are able to move under the influence of an electric field, they are the main transporters of electric current in metals. In this case, the movement of electrons does not lead to any chemical changes.
Charge transfer in molten salts or acid solutions is carried out by ions. They can be charged either positively or negatively. Unlike metals, the redistribution of charges in these liquids is accompanied by chemical reactions. Therefore, solutions are called conductors of the second kind, that is, those that, under the influence of direct currents, lead to a change in the chemical composition of the substance.
Thus, substances are conventionally divided according to the type of conductivity:
- conductors of the first kind (metals);
- conductors of the second kind (salt, alkaline and acid solutions);
- semiconductors (electron-hole conductivity);
- dielectrics (substances that are unable to conduct electricity due to the lack of free carriers).
Electrification of the body
Macroscopic bodies are, as a rule, electrically neutral. An atom of any substance is neutral because the number of electrons in it is equal to the number of protons in the nucleus. Positively and negatively charged particles are connected to each other by electrical forces and form neutral systems.
A large body is charged when it contains an excess number of elementary particles with the same charge sign. Negative
the charge of a body is due to the excess of electrons compared to protons, and
the positive
charge is due to their deficiency.
In order to obtain an electrically charged macroscopic body or, as they say, to electrify
it, you need to separate part of the negative charge from the positive charge associated with it.
The easiest way to do this is with friction. If you run a comb through your hair, a small part of the most mobile charged particles - electrons - will move from the hair to the comb and charge it negatively, and the hair will become positively charged. When electrified by friction, both bodies acquire charges of opposite sign, but equal in magnitude.
It is very simple to electrify bodies using friction. But explaining how this happens turned out to be a very difficult task.
1 version
. When electrifying bodies, close contact between them is important. Electrical forces hold electrons inside the body. But for different substances these forces are different. During close contact, a small part of the electrons of the substance in which the connection of electrons with the body is relatively weak passes to another body. The electron movements do not exceed the interatomic distances (10-8 cm). But if the bodies are separated, then both of them will be charged. Since the surfaces of bodies are never perfectly smooth, the close contact between bodies necessary for transition is established only on small areas of the surfaces. When bodies rub against each other, the number of areas with close contact increases, and thereby the total number of charged particles passing from one body to another increases. But it is not clear how electrons can move in such non-conducting substances (insulators) as ebonite, plexiglass and others. They are bound in neutral molecules.
2 version
. Using the example of an ionic LiF crystal (insulator), this explanation looks like this. During the formation of a crystal, various types of defects arise, in particular vacancies - unfilled spaces at the nodes of the crystal lattice. If the number of vacancies for positive lithium ions and negative fluorine ions is not the same, then the crystal will be charged in volume upon formation. But the charge as a whole cannot be retained by the crystal for long. There is always a certain amount of ions in the air, and the crystal will pull them out of the air until the charge of the crystal is neutralized by a layer of ions on its surface. Different insulators have different space charges, and therefore the charges of the surface layers of ions are different. During friction, the surface layers of ions are mixed, and when the insulators are separated, each of them becomes charged.
Can two identical insulators, for example the same LiF crystals, be electrified by friction? If they have the same own space charges, then no. But they can also have different own charges if the crystallization conditions were different and a different number of vacancies appeared. As experience has shown, electrification during friction of identical crystals of ruby, amber, etc. can actually occur. However, the above explanation is unlikely to be correct in all cases. If bodies consist, for example, of molecular crystals, then the appearance of vacancies in them should not lead to charging of the body.
Another way to electrify bodies is to expose them to various radiations.
(in particular, ultraviolet, x-ray and
γ
-radiation). This method is most effective for electrifying metals, when, under the influence of radiation, electrons are knocked out from the surface of the metal and the conductor acquires a positive charge.
Electrification through influence
.
The conductor is charged not only upon contact with a charged body, but also when it is at some distance. Let's explore this phenomenon in more detail. Let's hang light sheets of paper on an insulated conductor (Fig. 3). If the conductor is not charged at first, the leaves will be in the non-deflected position. Let us now bring an insulated metal ball, highly charged, to the conductor, for example, using a glass rod. We will see that the sheets suspended at the ends of the body, at points a
and
b
, are deflected, although the charged body does not touch the conductor.
The conductor was charged through influence, which is why the phenomenon itself was called “ electrification through influence
” or “
electrical induction
”.
Charges obtained through electrical induction are called induced
or
induced
.
The leaves suspended at the middle of the body, at points a
' and
b
', do not deviate.
This means that induced charges arise only at the ends of the body, and its middle remains neutral, or uncharged. By bringing an electrified glass rod to the sheets suspended at points a
and
b
, it is easy to verify that the sheets at point
b
repel from it, and the sheets at point
a
are attracted. This means that at the remote end of the conductor a charge of the same sign appears as on the ball, and on nearby parts charges of a different sign arise. By removing the charged ball, we will see that the leaves will go down. The phenomenon proceeds in a completely similar way if we repeat the experiment by charging the ball negatively (for example, using sealing wax).
Rice. 3
From the point of view of electronic theory, these phenomena are easily explained by the existence of free electrons in a conductor. When a positive charge is applied to a conductor, electrons are attracted to it and accumulate at the nearest end of the conductor. A certain number of “excess” electrons appear on it, and this part of the conductor becomes negatively charged. At the far end there is a lack of electrons and, therefore, an excess of positive ions: a positive charge appears here.
When a negatively charged body is brought close to a conductor, electrons accumulate at the far end, and an excess of positive ions is produced at the near end. After removing the charge that causes the movement of electrons, they are again distributed throughout the conductor, so that all parts of it are still uncharged.
The movement of charges along the conductor and their accumulation at its ends will continue until the influence of excess charges formed at the ends of the conductor balances the electrical forces emanating from the ball, under the influence of which the redistribution of electrons occurs. The absence of charge at the middle of the body shows that the forces emanating from the ball and the forces with which the excess charges accumulated at the ends of the conductor act on free electrons are balanced here.
Induced charges can be separated if, in the presence of a charged body, the conductor is divided into parts. Such an experience is depicted in Fig. 4. In this case, the displaced electrons can no longer return back after removing the charged ball; since there is a dielectric (air) between both parts of the conductor. Excess electrons are distributed throughout the left side; lack of electrons at point b
is partially replenished from the region of point
b
', so that each part of the conductor turns out to be charged: the left - with a charge opposite in sign to the charge of the ball, the right - with a charge of the same name as the charge of the ball.
Not only the leaves at points a
and
b
, but also the previously stationary leaves at points
a
' and
b
'.
Rice. 4
Charge interaction
Numerous experiments have shown that charged elementary particles interact with each other. Carriers of like charges repel, and carriers of unlike charges attract (see Fig. 5).
Rice. 5. Interaction of elementary particles
The force of interaction of point charges is determined by the formula resulting from Coulomb’s law: F = (k*q1*q2)/r2 , where q1 and q2 are two charged points located at a distance r, and k is a coefficient, the dimension of which depends on the chosen system measurements, and the value depends on the properties of the environment. Coulomb's law is one of the fundamental laws of physics.
Rice. 6. Interpretation of Coulomb's law
Electrical capacity. Capacitor
Electric capacitance (electric capacity) is a scalar physical quantity that characterizes the ability of an isolated conductor to hold an electric charge.
The designation is \( C \), the SI unit is farad (F).
A solitary conductor is a conductor that is distant from other conductors and charged bodies.
Farad is the electrical capacity of such an isolated conductor, the potential of which changes by 1 V when a charge of 1 C is imparted to it:
Formula for calculating electrical capacity:
where \( q \) is the charge of the conductor, \( \varphi \) is its potential.
Electrical capacity depends on its linear dimensions and geometric shape. Electrical capacity does not depend on the material of the conductor and its state of aggregation. The electrical capacity of a conductor is directly proportional to the dielectric constant of the medium in which it is located.
A capacitor is a system of two conductors separated by a dielectric layer, the thickness of which is small compared to the size of the conductors.
The conductors are called capacitor plates. The charges of the capacitor plates are equal in magnitude and opposite in sign of charge. The electric field is concentrated between the plates of the capacitor. Capacitors are used to store electrical charges.
The electrical capacity of the capacitor is calculated by the formula:
where \( q \) is the charge modulus of one of the plates, \( U \) is the potential difference between the plates.
The electrical capacity of a capacitor depends on the linear dimensions and geometric shape and the distance between the conductors. The electrical capacity of a capacitor is directly proportional to the dielectric constant of the substance between the conductors.
A flat capacitor consists of two parallel plates of area \( S \) located at a distance \( d \) from each other.
Electrical capacity of a flat capacitor:
where \( \varepsilon \) is the dielectric constant of the substance between the plates, \( \varepsilon_0 \) is the electrical constant.
On the electrical diagram the capacitor is designated:
Types of capacitors:
- by type of dielectric - air, paper, etc.;
- shape – flat, cylindrical, spherical;
- by electrical capacity - constant and variable capacity.
Capacitors can be connected to each other.
Parallel connection of capacitors
In a parallel connection, capacitors are connected by similarly charged plates. The capacitor voltages are:
Total capacity:
Series connection of capacitors
When capacitors are connected in series, their oppositely charged plates are connected.
The charges of the capacitors with this connection are equal:
Total voltage:
The reciprocal of the total capacity:
With this connection, the total capacitance is always less than the capacitances of the individual capacitors.
Important! If a capacitor is connected to a current source, then the potential difference between its plates does not change when the electrical capacitance changes and is equal to the source voltage. If a capacitor is charged to a certain potential difference and is disconnected from the current source, then its charge does not change when the electrical capacitance changes.
Application of capacitors Capacitors are used in electronic devices as charge storage devices to smooth out ripples in AC rectifiers.
Law of conservation of electric charge
It has been experimentally established that in a closed system one of the fundamental laws of physics is satisfied - the conservation law. In an isolated system, the total charge does not disappear, but persists over time. In addition, it is quantized, that is, it changes in portions that are multiples of the charge of the elementary particle.
The algebraic sum of charges is a constant value: q1 + q2 + … + qn = const (see Fig. 7).
Rice. 7. Preserve static electricity
The law was formulated by B. Franklin (1747) and confirmed by M. Faraday in 1843.
Literature
- Aksenovich L. A. Physics in secondary school: Theory. Tasks. Tests: Textbook. allowance for institutions providing general education. environment, education / L. A. Aksenovich, N. N. Rakina, K. S. Farino; Ed. K. S. Farino. - Mn.: Adukatsiya i vyakhavanne, 2004. - P. 209-210, 211-214.
- Zhilko, V.V. Physics: textbook. allowance for 11th grade. general education institutions with Russian language training with a 12-year period of study (basic and advanced levels) /V. V. Zhilko, L. G. Markovich. — 2nd ed., revised. — Minsk: Nar. Asveta, 2008. - pp. 72-79.
Measurement methods
The simplest measuring device is an electroscope. It consists of two foil petals located on a metal rod. The structure is covered with a glass cover.
If you touch the rod with an electrified body, the petals become electrified. Since the signs on them are the same, the Coulomb force will push them in different directions. By the magnitude of the deflection angle, you can estimate the amount of static electricity received by the petals.
A more complex device is an electrometer (schematic representation in Fig. 8). The device consists of an electrometer rod, a pointer and a scale. The principle of operation is similar to an electroscope (the arrow is repelled from the rod). Thanks to the presence of a scale, the deflection of the electrometer needle shows the quantitative value of the transferred electricity.
Rice. 8. Schematic representation of an electrometer
We have already mentioned that Coulomb used torsion balances in his experiments. This measuring device allowed the scientist to discover the famous law named after him.