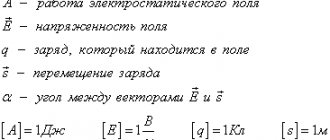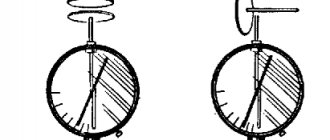Home Online textbooks Database of Russian tutors Physics simulators Preparation for the Unified State Exam 2022 online
Chapter 1. Electrodynamics
Many physical phenomena observed in nature and the life around us cannot be explained only on the basis of the laws of mechanics, molecular kinetic theory and thermodynamics. These phenomena manifest forces acting between bodies at a distance, and these forces do not depend on the masses of the interacting bodies and, therefore, are not gravitational. These forces are called electromagnetic forces.
The ancient Greeks knew about the existence of electromagnetic forces. But the systematic, quantitative study of physical phenomena in which the electromagnetic interaction of bodies is manifested began only at the end of the 18th century. Through the work of many scientists in the 19th century, the creation of a harmonious science studying electrical and magnetic phenomena was completed. This science, which is one of the most important branches of physics, is called electrodynamics.
The main objects of study in electrodynamics are electric and magnetic fields created by electric charges and currents.
1.1. Electric charge. Coulomb's law
Like the concept of gravitational mass of a body in Newtonian mechanics, the concept of charge in electrodynamics is the primary, basic concept.
Electric charge is a physical quantity that characterizes the property of particles or bodies to enter into electromagnetic force interactions.
Electric charge is usually denoted by the letters q or Q.
The totality of all known experimental facts allows us to draw the following conclusions:
- There are two types of electric charges, conventionally called positive and negative.
- Charges can be transferred (for example, by direct contact) from one body to another. Unlike body mass, electric charge is not an integral characteristic of a given body. The same body under different conditions can have a different charge.
- Like charges repel, unlike charges attract. This also reveals the fundamental difference between electromagnetic forces and gravitational ones. Gravitational forces are always attractive forces.
One of the fundamental laws of nature is the experimentally established law of conservation of electric charge.
In an isolated system, the algebraic sum of the charges of all bodies remains constant:
| q1 + q2 + q3 + … +qn = const. |
The law of conservation of electric charge states that in a closed system of bodies processes of creation or disappearance of charges of only one sign cannot be observed.
From a modern point of view, charge carriers are elementary particles. All ordinary bodies are composed of atoms, which include positively charged protons, negatively charged electrons and neutral particles - neutrons. Protons and neutrons are part of atomic nuclei, electrons form the electron shell of atoms. The electric charges of a proton and an electron are exactly the same in magnitude and equal to the elementary charge e.
In a neutral atom, the number of protons in the nucleus is equal to the number of electrons in the shell. This number is called the atomic number. An atom of a given substance may lose one or more electrons or gain an extra electron. In these cases, the neutral atom turns into a positively or negatively charged ion.
Charge can be transferred from one body to another only in portions containing an integer number of elementary charges. Thus, the electric charge of a body is a discrete quantity:
Physical quantities that can only take a discrete series of values are called quantized. Elementary charge e is a quantum (smallest portion) of electric charge. It should be noted that in modern physics of elementary particles the existence of so-called quarks is assumed - particles with a fractional charge and However, quarks have not yet been observed in a free state.
In ordinary laboratory experiments, an electrometer is used to detect and measure electrical charges - a device consisting of a metal rod and a pointer that can rotate around a horizontal axis (Fig. 1.1.1). The arrow rod is isolated from the metal body. When a charged body comes into contact with the electrometer rod, electric charges of the same sign are distributed over the rod and the pointer. Electrical repulsion forces cause the needle to rotate through a certain angle, by which one can judge the charge transferred to the electrometer rod.
| Figure 1.1.1. Transfer of charge from a charged body to an electrometer |
The electrometer is a rather crude instrument; it does not allow one to study the forces of interaction between charges. The law of interaction of stationary charges was first discovered by the French physicist C. Coulomb in 1785. In his experiments, Coulomb measured the forces of attraction and repulsion of charged balls using a device he designed - a torsion balance (Fig. 1.1.2), which was distinguished by extremely high sensitivity. For example, the balance beam was rotated by 1° under the influence of a force of the order of 10–9 N.
The idea of the measurements was based on Coulomb's brilliant guess that if a charged ball is brought into contact with exactly the same uncharged one, then the charge of the first will be divided equally between them. Thus, a way was indicated to change the charge of the ball by two, three, etc. times. In Coulomb's experiments, the interaction between balls whose dimensions were much smaller than the distance between them was measured. Such charged bodies are usually called point charges.
A point charge is a charged body whose dimensions can be neglected in the conditions of this problem.
| Figure 1.1.2. Coulomb device |
| Figure 1.1.3. Interaction forces between like and unlike charges |
Based on numerous experiments, Coulomb established the following law:
The interaction forces between stationary charges are directly proportional to the product of the charge moduli and inversely proportional to the square of the distance between them:
Interaction forces obey Newton's third law: They are repulsive forces with the same signs of charges and attractive forces with different signs (Fig. 1.1.3). The interaction of stationary electric charges is called electrostatic or Coulomb interaction. The branch of electrodynamics that studies the Coulomb interaction is called electrostatics.
Coulomb's law is valid for point charged bodies. In practice, Coulomb's law is well satisfied if the sizes of charged bodies are much smaller than the distance between them.
The proportionality coefficient k in Coulomb's law depends on the choice of system of units. In the International SI system, the unit of charge is the coulomb (C).
A coulomb is a charge passing through the cross section of a conductor in 1 s at a current of 1 A. The unit of current (ampere) in SI is, along with units of length, time and mass, the basic unit of measurement.
The coefficient k in the SI system is usually written as:
where is the electrical constant.
In the SI system, the elementary charge e is equal to:
| e = 1.602177·10–19 C ≈ 1.6·10–19 C. |
Experience shows that the Coulomb interaction forces obey the principle of superposition.
If a charged body interacts simultaneously with several charged bodies, then the resulting force acting on a given body is equal to the vector sum of the forces acting on this body from all other charged bodies.
Rice. 1.1.4 explains the principle of superposition using the example of the electrostatic interaction of three charged bodies.
| Figure 1.1.4. The principle of superposition of electrostatic forces |
| Model. Interaction of point charges |
The principle of superposition is a fundamental law of nature. However, its use requires some caution when we are talking about the interaction of charged bodies of finite sizes (for example, two conducting charged balls 1 and 2). If a third charged ball is brought to a system of two charged balls, then the interaction between 1 and 2 will change due to the redistribution of charges.
The principle of superposition states that for a given (fixed) distribution of charges on all bodies, the forces of electrostatic interaction between any two bodies do not depend on the presence of other charged bodies.
Stationary point charges
Coulomb's law applies to stationary bodies whose size is much less than their distance from other objects. A point electric charge is concentrated on such bodies. When solving physical problems, the dimensions of the bodies under consideration are neglected, because they don't really matter.
In practice, point charges at rest are depicted as follows:
In this case, q1 and q2 are positive electric charges, and they are acted upon by the Coulomb force (not shown in the figure). The size of the point objects does not matter.
Note! Charges at rest are located from each other at a given distance, which in problems is usually denoted by the letter r. Further in the article we will consider these charges in a vacuum.
Torsion scales by Charles Coulomb
This device, developed by Coulomb in 1777, helped to derive the dependence of the force that was later named in his honor. With its help, the interaction of point charges, as well as magnetic poles, is studied.
A torsion balance has a small silk thread placed in a vertical plane from which a balanced lever hangs. Point charges are located at the ends of the lever.
Under the influence of external forces, the lever begins to move horizontally. The lever will move in the plane until it is balanced by the elastic force of the thread.
During the movement, the lever deviates from the vertical axis by a certain angle. It is taken as d and called the angle of rotation. Knowing the value of this parameter, you can find the torque of the resulting forces.
Direction of the Coulomb force and vector form of the formula
To fully understand the formula, Coulomb’s law can be depicted visually:
F1,2 is the force of interaction of the first charge relative to the second.
F2,1 is the interaction force of the second charge relative to the first.
Also, when solving problems of electrostatics, it is necessary to take into account an important rule: like electric charges repel, and opposite ones attract. The location of the interaction forces in the figure depends on this.
If unlike charges are considered, then the forces of their interaction will be directed towards each other, representing their attraction.
The formula of the basic law of electrostatics in vector form can be represented as follows:
is the force acting on a point charge q1 from the side of charge q2,
is the radius vector connecting charge q2 with charge q1,
What is an electric field
One day, Benjamin Franklin, whose portrait can be seen on the hundred dollar bill, was flying a kite during a rain and thunderstorm. He chose such a strange activity for a reason, but in order to study the nature of lightning. Noticing that the hairs on the wet cord rose up (that is, it became electrified), Franklin wanted to touch the metal key. But as soon as he brought his finger closer, a characteristic crack was heard and sparks appeared. The electric field was activated.
This happened in the middle of the 18th century, but for another century scientists could not really explain how exactly charged bodies interact with each other without touching. Michael Faraday was the first to discover that there was some kind of intermediate link between them. His conclusions were confirmed by James Maxwell, who found that it takes time for one such object to influence another, which means they interact through an “intermediary.”
In modern physics, an electric field is a kind of matter that arises between charged bodies and determines their interaction. When it comes to stationary objects, the field is called electrostatic.
Objects carrying like charges will repel, and objects with unlike charges will attract.
Determination of electric field strength
To study the electric field, point charges are used. Let's find out what it is.
A point charge is an electrified object whose dimensions can be neglected because it is too small compared to the distance separating this object from other charged bodies.
Now let's talk directly about intensity, which is one of the main characteristics of the electric field. This is a vector physical quantity. Unlike scalar ones, it has not only a meaning, but also a direction.
In order to study the electrical intensity, you need to place another point charge q2 in the field of a charged body q1 (let's say they are both positive). A certain force will act on q2 from q1. Obviously, for calculations, you need to keep in mind both the value of a given force and its direction, that is, a vector.
Electric field strength is an indicator equal to the ratio of the force acting on a charge in an electric field to the magnitude of this charge.
Tension is a force characteristic of the field. It tells us how strong the influence of the field at a given point is not only on another charge, but also on living and inanimate objects.