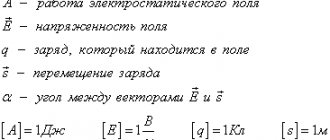A direct electric current occurs if there is an electric field (potential difference) and free charge carriers: positive and negative particles or quasiparticles. An atom cannot be a free charge carrier; it is electrically neutral; but if an electron or several electrons leave their orbits, negative and positive charge carriers appear. To remove an electron from an atom, energy must be applied; it can be thermal, electrical, mechanical energy, chemical reaction.
The structure of an atom affects the electrical conductivity of a substance
An atom is a massive positive nucleus and an electron cloud. According to the laws of the microworld, the electron cloud of an atom cannot be homogeneous and chaotic. It is divided into so-called shells, where the hierarchy of quantum states is strictly observed.
The properties of an atom depend on how many electrons are in the outer shell. The outer electron shell of noble gases is completely filled. Under normal conditions they do not conduct current. But if special conditions are created (low pressure, high electrical voltage), atoms lose electrons and an electric current arises.
Metal atoms have from one to three electrons in their outer shell, and these outer electrons inside the crystal lattice of the metal easily “fly” from their orbits and form an “electron gas” that ensures good electrical conductivity of metals.
For the occurrence of an electric current in a metal, no additional conditions are necessary: the electron gas reacts to the slightest electrical voltage, measured in millivolts, microvolts. Moreover, metals are also suitable for transmitting high currents and voltages.
Elements with more than three electrons in their outer shell are nonmetals, with the presence of four electrons giving the elements a special transition status. They conduct current, but much weaker than metals, and their conduction mechanism differs from the “metallic” one.
Nonmetals that have five or more electrons in their outer shell form a group of dielectrics. Their conductivity under normal conditions, at low voltages, is very small. Among them there are substances from which reliable insulators are made. However, for each insulator the electrical strength is indicated: the maximum voltage that it can withstand. Exceeding this voltage leads to breakdown of the insulator: electrons are torn from their orbits, atoms are destroyed, and a channel with high electrical conductivity appears in the dielectric.
Types of electric current
There are two types of current: direct current and alternating current.
Constant
It is characterized by an invariable direction of movement of charged particles. Examples are dry batteries, small-capacity batteries, solar panels, etc. They are used in the process of arc welding, when organizing traffic on electrified railway tracks, aluminum electrolysis, etc. They are formed using special generators. Its direction is taken to be the movement of particles from “plus” to “minus”.
DC motor
Variable
In this case, it is able to change its characteristics: the magnitude and direction of movement. The number of changes per unit time is called frequency, measured in hertz. It is used on construction sites and for industrial purposes (grinding equipment, electric drills, etc.)
Electrons, protons, ions
Particles that carry a negative charge are electrons. Protons have a positive charge. The charges of electrons and protons are opposite in sign, but exactly equal in magnitude. But these particles differ greatly in mass: protons are 1800 times heavier than electrons.
Mobile, nimble electrons are free charge carriers in many substances: metals, semiconductors, and gaseous media.
Protons, as individual particles, are free charge carriers only in high-temperature plasma. Under normal conditions, positively charged free charge carriers exist in the form of ions. An ion is an atom or group of atoms that has fewer electrons in its electron cloud than protons in its nuclei.
Ions can be free charge carriers in liquid and gaseous media. In a solid, ionized atoms are firmly held in a crystal lattice.
2.1. Types of electrical conductivity of semiconductors.
The operating principle of semiconductor devices is due to the fact that in semiconductors there are two types of electrical conductivity - electronic and hole.
Electronic conductivity is caused by the movement of free electrons in one direction. At ordinary operating temperatures, in pure, unalloyed semiconductors there are always electrons that are very weakly bound to the nuclei of atoms, become free and perform random, chaotic, thermal movement between the atoms of the crystal lattice. These electrons, under the influence of an electric field, can begin to move in a certain direction. This directed movement is an electric current.
Hole electrical conductivity is a feature of semiconductors and is not observed in metals, i.e. in conductor materials (conductors).
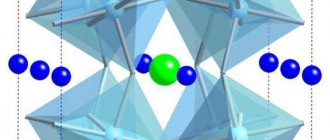
In a semiconductor atom, under the influence of thermal or other external influences, one of the valence electrons can leave the atom and become free (Fig. 3). Then the atom acquires a positive charge, but in magnitude equal to the negative charge of the electron. Such an atom is called a positive ion, and the process of converting an atom into an ion is called ionization.
In semiconductors, the crystal lattice is strong. Its ions do not move, but remain in their places at the nodes of the crystal lattice, i.e. are stationary charges.
The absence of an electron in the orbit of a semiconductor atom is conventionally called a hole . This emphasizes that the atom is missing one electron, i.e., a free space has formed - a hole. Holes [3] behave like elementary positive charges.
The appearance of a hole can be explained using a planar model of a semiconductor (Fig. 3). One of the electrons participating in the covalent bond, having received additional energy W > ?W, becomes a conduction electron , i.e., a free charge carrier. It can move between the atoms of the crystal lattice, and when a large number of such free electrons move in one direction, they create an electric current. His old place is now vacant. This is the hole shown in Fig. 3 with a light circle.
In hole conductivity, holes move under the influence of an applied potential difference, which is equivalent to the movement of positive charges. Such a process is shown in Fig. 4, which shows several atoms located along the semiconductor at different times.
Suppose that at the initial moment of time (Fig. 4, a) a hole appeared in the leftmost atom (number 1), due to the fact that an electron left this atom, i.e. became free.
The atom with the hole (shaded) has a positive charge and can attract an electron from neighboring atom number 2.
If an electric field (potential difference) operates in a semiconductor, then this field tends to move electrons in the direction from a negative potential to a positive one. Therefore, at the next moment (Fig. 4b), one electron from atom 2 will move to atom 1 and fill the hole, and a new hole will be formed in atom 2. Next, one electron from atom 3 will move to atom 2 and fill the hole in it. Then a hole will appear in atom 3 Fig. 4 (Fig. 4, c), etc.
This process will continue, and the hole will move from the leftmost atom 1 to the rightmost atom number 6. In other words, the positive charge initially arising in atom 1 will move to atom 6 (Fig. 4, f).
As can be seen, with hole electrical conductivity, electrons actually also move, but more limitedly, i.e. the distance traveled is less than in electron conduction, where the electron can move in the crystal lattice. Rice. 5
With hole electrical conductivity, electrons move from these atoms only to neighboring ones. The result of this is the movement of positive charges - holes in the direction opposite to the movement of electrons.
The electrical conductivity of semiconductors can also be explained by their energy diagram (Fig. 5).
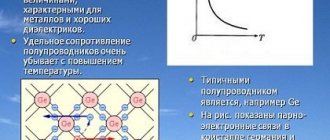
At temperature absolute zero, i.e. Т = 0К = – 273ºС, a semiconductor that does not contain impurities is a dielectric; it does not contain electrons or conduction holes. But with increasing temperature T > 0K, the electrical conductivity of the semiconductor increases, since the electrons of the valence band receive additional energy during heating [4] W = kT and due to this, a certain number of them overcome the band gap and move from the valence band to the conduction band. This transition is shown in Fig. 6 by a solid arrow.
The band gap ΔW of semiconductors is relatively small (for germanium ΔW = 0.72 eV, and for silicon ΔW = 1.12 eV).
Thus, conduction electrons and electronic conductivity occurs. Each electron that moves into the conduction band leaves a free space in the valence band—a hole, i.e., conduction holes , the number of which is equal to the number of electrons that moved into the conduction band. Consequently, along with electron conductivity, hole electrical conductivity is also created.
Conductivity of metals: electron gas
The crystal lattice of a metal creates internal forces that encourage electrons from the outer shells to leave “their” core. For their transition to the free state, the transition to the conduction band, no additional effort is required.
If an electric field is applied to a metal object, a current will flow. Electric current is the total electric charge passing through the cross section of a conducting body per second.
Electrons move along the entire length of the conductor; the measured current values are the same in any section.
Note that current instantaneously arises throughout the conductor due to the propagation of the electric field, but free charge carriers, electrons, move very slowly.
At low voltages, they barely wander, bumping into atomic nuclei; their thermal movement is much more intense than their ordered movement to the positive electrode.
If the metal is heated, the current will decrease. This is because the thermal movement of the electrons themselves and the crystal lattice slows down the ordered movement of electrons, in other words, as the temperature increases, the electrical resistance of the metal increases, and its conductivity decreases.
Electric current increases the temperature of the metal, which is associated with an initial jump in the current in the conductor and its subsequent decrease. Cold metal has little resistance and allows significant current to pass; but this current heats the metal, the resistance increases, and the current decreases.
The directed movement of electric charges is called electric current . Depending on the type of conductor, charge carriers can be electrons and ions. In metal conductors these are free electrons, or conduction electrons ; in galvanic baths, i.e. in electrolyte solutions, these are positive and negative ions. Bodies or substances in which electric current can be created are called conductors of electric current. All metals, aqueous solutions of salts or acids, and ionized gases are conductors.
When free charged particles move, charge transfer occurs. A quantitative characteristic - the strength of $$ I$$ current - is considered to be the rate of charge transfer through any cross section of a conductor, i.e. the amount of charge moved through the “control surface” , on which the charge that has crossed it is calculated, per unit time:
`I=q/t`, (1)
where `q` is the charge that passed through an arbitrary fixed cross-section of the conductor during the time from `0` to `t`. If the current does not change over time, the current is called constant . The SI unit of current is called the ampere (A) (in honor of A.M. Ampere, a 19th-century French scientist) and is introduced through the magnetic interaction of currents.
One ampere is the strength of such a current maintained in two infinite (very long) straight parallel conductors of negligibly small cross-sectional area, located at a distance of `1` m in a vacuum, at which, calculated per `1` meter of the length of the conductor, the force `F= 2*10^(-7) "Н"`.
The unit of current, the ampere, along with the meter, second, and kilogram, is the basic unit of the SI system. The charge unit coulomb (C) is a derivative and is introduced in accordance with (1): one coulomb is an electric charge passing through the cross section of a conductor at a current strength of $$ 1\mathrm{A}$$ for $$ 1\mathrm{ c}$$, i.e. $$ 1\mathrm{Cl}=1\mathrm{A}·1\mathrm{c}.$$
The direction of electric current to be the direction in which positively charged current carriers move.
The ratio of current strength `I` to the cross-sectional area `S` of the conductor is called current density:
`j=I/S`, (2)
which is equal to the current strength per unit cross-sectional area.
Example 6
A direct current flows through the wire. A charge of `q=1.2` C flowed through an arbitrary cross-section during a time `t=2` min. Find the current strength `I` in the wire and its density `j`. The cross-sectional area of the conductor is `S=0.5 "mm"^2`.
Solution
We determine the current strength using formula (1):
$$ I={\displaystyle \frac{q}{t}}={\displaystyle \frac{\mathrm{1,2}}{120}}=\mathrm{0,01}\mathrm{A}$$ ,
We find the current density using formula (2):
`j=I/S=(0.01)/(0.5*10^(-6))=2*10^4″А»//»м»^2`.
Example 7
According to the model proposed by Niels Bohr, in the ground state of the hydrogen atom, an electron moves around a stationary proton in a circular orbit of radius `r=0.53*10^(-10)` m with a speed of `v=2.2*10^6` m /With. What value of current `I` is equivalent to the motion of an electron in orbit? What is the direction of this current? Elementary charge `e=1.6*10^(-19)` Cl.
Solution
In the model under consideration, an electron orbits a proton with a period of `T=(2pir)/v`. In `t=1` s, the electron will cross any control surface on which the transferred charge is calculated, `nu=1/T` times. Then the charge `q=e*nu` will pass through this surface in `t=1` s, i.e. the strength of the equivalent current in accordance with (1) is equal
`I=q/t=enu=ev/(2pir)=1.6*10^(-19) *(2.2*10^6)/(2*3.14*0.53*10^( -10))~~1.06*10^(-3) “A”`.
Since an electron is a negatively charged particle, the direction of the current in question is opposite to the direction of motion of the electrons.
Current in gas discharge lamps
Atoms of noble gases are strong and do not tend to enter into chemical reactions and give up electrons. But under the influence of an electric field, the electron cloud is deformed, stretched... and some electrons fly out of orbit.
If the conductivity of inert gases was determined only by these electrons, flown from orbits under the influence of an electric field, then gas-discharge lamps would not glow at relatively low electrical voltages. But there are much more conduction electrons in argon, neon, and helium lamps!
Electrons that have gained freedom are accelerated under the influence of an electric field and collide with neutral atoms, and new electrons are often “knocked out” from orbit.
These new electrons are also accelerated and contribute to the ionization of new atoms. The process takes on the character of an avalanche, and the current increases almost instantly.
In a gas-discharge lamp there are both negative charge carriers (electrons) and positive charge carriers (ionized atoms). The ions move to the negative electrode and are neutralized, gaining the missing electrons; electrons fly to the positive electrode.
An avalanche-like increase in the number of free electrons also occurs in the air during a lightning discharge.
2.1. Types of electrical conductivity of semiconductors.
The operating principle of semiconductor devices is due to the fact that in semiconductors there are two types of electrical conductivity - electronic and hole.
Electronic conductivity is caused by the movement of free electrons in one direction. At ordinary operating temperatures, in pure, unalloyed semiconductors there are always electrons that are very weakly bound to the nuclei of atoms, become free and perform random, chaotic, thermal movement between the atoms of the crystal lattice. These electrons, under the influence of an electric field, can begin to move in a certain direction. This directed movement is an electric current.
Hole electrical conductivity is a feature of semiconductors and is not observed in metals, i.e. in conductor materials (conductors).
In a semiconductor atom, under the influence of thermal or other external influences, one of the valence electrons can leave the atom and become free (Fig. 3). Then the atom acquires a positive charge, but in magnitude equal to the negative charge of the electron. Such an atom is called a positive ion, and the process of converting an atom into an ion is called ionization.
In semiconductors, the crystal lattice is strong. Its ions do not move, but remain in their places at the nodes of the crystal lattice, i.e. are stationary charges.
The absence of an electron in the orbit of a semiconductor atom is conventionally called a hole . This emphasizes that the atom is missing one electron, i.e., a free space has formed - a hole. Holes [3] behave like elementary positive charges.
The appearance of a hole can be explained using a planar model of a semiconductor (Fig. 3). One of the electrons participating in the covalent bond, having received additional energy W > ?W, becomes a conduction electron , i.e., a free charge carrier. It can move between the atoms of the crystal lattice, and when a large number of such free electrons move in one direction, they create an electric current. His old place is now vacant. This is the hole shown in Fig. 3 with a light circle.
In hole conductivity, holes move under the influence of an applied potential difference, which is equivalent to the movement of positive charges. Such a process is shown in Fig. 4, which shows several atoms located along the semiconductor at different times.
Suppose that at the initial moment of time (Fig. 4, a) a hole appeared in the leftmost atom (number 1), due to the fact that an electron left this atom, i.e. became free.
The atom with the hole (shaded) has a positive charge and can attract an electron from neighboring atom number 2.
If an electric field (potential difference) operates in a semiconductor, then this field tends to move electrons in the direction from a negative potential to a positive one. Therefore, at the next moment (Fig. 4b), one electron from atom 2 will move to atom 1 and fill the hole, and a new hole will be formed in atom 2. Next, one electron from atom 3 will move to atom 2 and fill the hole in it. Then a hole will appear in atom 3 Fig. 4 (Fig. 4, c), etc.
This process will continue, and the hole will move from the leftmost atom 1 to the rightmost atom number 6. In other words, the positive charge initially arising in atom 1 will move to atom 6 (Fig. 4, f).
As can be seen, with hole electrical conductivity, electrons actually also move, but more limitedly, i.e. the distance traveled is less than in electron conduction, where the electron can move in the crystal lattice. Rice. 5
With hole electrical conductivity, electrons move from these atoms only to neighboring ones. The result of this is the movement of positive charges - holes in the direction opposite to the movement of electrons.
The electrical conductivity of semiconductors can also be explained by their energy diagram (Fig. 5).
At temperature absolute zero, i.e. Т = 0К = – 273ºС, a semiconductor that does not contain impurities is a dielectric; it does not contain electrons or conduction holes. But with increasing temperature T > 0K, the electrical conductivity of the semiconductor increases, since the electrons of the valence band receive additional energy during heating [4] W = kT and due to this, a certain number of them overcome the band gap and move from the valence band to the conduction band. This transition is shown in Fig. 6 by a solid arrow.
The band gap ΔW of semiconductors is relatively small (for germanium ΔW = 0.72 eV, and for silicon ΔW = 1.12 eV).
Thus, conduction electrons and electronic conductivity occurs. Each electron that moves into the conduction band leaves a free space in the valence band—a hole, i.e., conduction holes , the number of which is equal to the number of electrons that moved into the conduction band. Consequently, along with electron conductivity, hole electrical conductivity is also created.
Electric current in liquids. Electrolysis.
Liquids can be either conductive or non-conducting. Machine oil has no electrical conductivity; gasoline and kerosene do not conduct current. But water is a good conductor of electric current, and the more salts dissolved in it, the lower its resistance.
Distilled water has a small amount of free charges, since a small proportion of water molecules are in a “disassembled state”: a positively charged hydrogen ion (in fact, just a proton), and a negatively charged group of hydrogen and oxygen atoms, which has an “extra” electron in its orbit.
If electrodes are immersed in a vessel with water, gas bubbles will begin to release. It happens like this: hydrogen ions move to the negative electrode, where the proton receives an electron for its shell, a full-fledged atom appears, which reunites with another hydrogen atom, forming a molecule. Hydrogen gas is released from the water.
(OH-) groups - an ionized pair of oxygen and hydrogen atoms - move towards the positive electrode. There they lose an extra electron, and either capture a hydrogen atom from another group and turn into a water molecule, or disintegrate - the hydrogen complements another water molecule, and the oxygen atom reunites with another similar one and floats to the top. This is how oxygen and hydrogen are produced by electrolysis of water.
Pure, distilled water is a weak conductor. But if ordinary table salt is dissolved in it, the conductivity increases several times. Salt molecules in an aqueous solution break down into ions: positive sodium and negative chlorine. The passage of current is accompanied by the release of pure sodium on the negative electrode, and poisonous chlorine gas on the positive electrode.
Electrolysis plays a big role in industry: it is used to coat objects with a metal film to obtain pure metals. But this is a harmful production, it affects the health of workers and the state of the environment. Recently, it has been replaced by other, more advanced technologies.
How is electricity directed (movement)
Current movement can occur in two ways. The direction of movement of charged particles is associated with the movement of electrons having a positive charge. When the current arises due to negative electrons, then the direction is taken opposite to their movement. This is typical for metal conductors. But current can arise in both liquid and gas, in which particles move freely along any trajectory due to the lack of a strong connection between them. In this case, the current carriers will be positive ions and negative electrons, and the electric current goes from “plus” to “minus”.
You might be interested in: Features of using rosin
Where is electric current used?
Today there is not a single area where electric power is not used. current in its various manifestations. Basically, alternating current is widely used: power supply, railway communication, lighting, various network equipment, etc. Direct current is used in electrolysis, welding, on-board systems of cars, and medicine.
For several centuries, scientists have been trying to find an answer to the question of why and how electric current occurs. Today the life of modern society cannot be imagined without it. The key to the successful use of electricity will be compliance with the basic safety rules associated with its use, since electricity, along with its benefits, can cause harm to a person and his health.