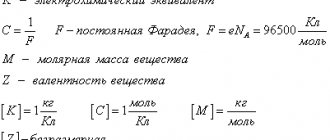Electric current in liquids
Like solids, liquids can be dielectrics and conductors. Distilled water, for example, is a dielectric, and a small amount of sodium chloride NaCl (also a dielectric) added to distilled water makes it a conductor.
This is explained as follows. In distilled water, the concentration of free charges is very small, so it conducts current poorly. The dielectric constant of water is ε = 81, therefore, when a substance is dissolved in water, the Coulomb forces of interaction between ions in the salt molecule decrease. And the energy of thermal (random) movement of particles may be enough for the molecule to break up into Na+ and Cl– ions.
- The breakdown of the molecules of a substance into ions when it is dissolved in a liquid is called electrolytic dissociation
.
The theory of electrolytic dissociation was developed in 1887 by the German scientist R. Clausius and the Swedish chemist S. Arrhenius.
Molecules of different substances dissociate differently and can split into two or more ions. The nature of dissociation is closely related to the chemical properties of the substance.
For example, when copper sulfate salt is dissolved in water, the CuSO4 molecule dissociates into two ions: Cu2+ and SO42-:
\(~CuSO_4 \leftrightarrows Cu^{2+} + SO_4^{2-}.\)
In the absence of an external electric field, the ions are in thermal chaotic motion.
When ions of opposite sign meet, they can again form a neutral molecule. This process is called recombination
ions (the reverse process of dissociation). Under constant conditions, a dynamic equilibrium is established in the solution, when the number of molecules that disintegrate into ions per second is equal to the number of pairs of ions that, at the same time, recombine into neutral molecules.
Degree of dissociation
α is determined by the ratio of the number of molecules disintegrated into ions to their total number. The degree of dissociation depends on temperature, solution concentration and dielectric constant of the solvent. Since the energy of thermal motion of molecules increases with increasing temperature, the degree of dissociation of the electrolyte increases and, consequently, the concentration of positively and negatively charged ions increases.
Let two electrodes, which are metal conductors, be placed in a vessel with an electrolyte solution, to which we connect an EMF source. The electrode connected to the positive terminal of the source is called the anode
, to the negative terminal -
cathode
.
An electric field will arise in the vessel, and negative ions (anions) will begin to move towards the anode, and positive ions (cations) - towards the cathode (Fig. 1). As a result, an electric current will be established in the electrolyte solution. Rice. 1 The term “ion” is translated from Greek as “going.” This is where the names “ anion
” — going to the anode, and “
cation
” — going to the cathode — come from.
- Electric current in liquids
is the directed movement of ions of both signs.
Since charge transfer in electrolytes is carried out by ions, such conductivity is called ionic
.
However, some liquids can also exhibit electronic conductivity
. Liquid metals, for example, have such conductivity.
- Liquids that conduct electric current are called electrolytes
.
For electrolytes, Ohm's law and the Joule-Lenz law are also valid.
In ionic conduction, the passage of current is associated with the transfer of matter. At the electrodes, substances that make up the electrolytes are released. At the anode, negative ions give up their extra electrons (this is called an oxidation reaction), and at the cathode, positive ions receive the missing electrons (a reduction reaction). By giving or receiving electrons, ions become neutral atoms. These atoms (or molecules formed from them) are released on the electrodes.
The resulting atoms can react with the electrodes or solvent.
Chemical reactions into which neutralized ions enter are called secondary
.
- The phenomenon of the release of substances on the electrodes when an electric current passes through the electrolyte is called electrolysis
.
A necessary condition for electrolysis is the passage of a direct electric current through the electrolyte.
Electrolysis was first observed in 1800 by W. Nicholson and A. Carlyle, who decomposed water using direct current. After 7 years, G. Davy isolated and discovered sodium using electrolysis.
Faraday's experiments and electrolysis
The flow of electric current in liquid substances is a product of the movement of charged ions. Problematic issues associated with the appearance and distribution of electric current in liquid substances were the basis for the research of the famous English scientist, experimental physicist and chemist Michael Faraday. Michael Faraday, thanks to numerous practical experiments, was able to find evidence that the weight of the substance released during electrolysis depends on time and electricity.
Is it difficult to figure it out on your own?
Try asking your teachers for help
Solving problems Tests Essays
At the same time, how long the experiments take is of great importance. In particular, the scientist was able to clarify the issue that during electrolysis, the separation of a certain substance requires the same number of electrical charges. This number was clearly defined and registered in a constant, which was called the Faraday number. In liquid substances, electric current has different distribution conditions.
Electric current interacts with molecular compounds of water. Water molecules significantly hinder all movements of ions, and this was not observed in experiments using an ordinary metal conductor. Thus, the formation of electric current during electrolytic reactions will not be so large. But with increasing temperature parameters of the liquid substance, the conductivity begins to increase over time. This means that the voltage of the electric current increases.
In particular, during electrolysis it was noted that the probability of a certain molecule breaking up into negative or positive ion charges increases due to the large number of molecules of the liquid used. When the liquid is saturated with ions above certain standards, the opposite process occurs. The conductivity of the liquid substance becomes lower again.
Today, the electrolysis process has found its use in most sectors of life, as well as in the scientific community and in production processes. Industrial organizations use electrolysis in the production and processing of metal and metal alloys. Electrochemical reactions take part in:
- Polishing planes.
- Electrolysis of salts.
- Electroplating.
- Other oxidation and reduction works.
Did not you find what you were looking for?
Just write and we will help
Laws of electrolysis
Electrolysis is described by two basic laws, experimentally established by Faraday in 1833-1834.
Faraday's first law
:
- the mass of substance m released on one of the electrodes is directly proportional to the charge Δ q
passed through the electrolyte:
m = K⋅Δq = K⋅I
⋅Δ
t
.
Here I
- current strength in the electrolyte, Δ
t
- time of current flow through the electrolyte,
K
- electrochemical equivalent of the substance.
- The electrochemical equivalent
is numerically equal to the mass of the substance
m
released on the electrode when a charge
q
of 1 C passes through the electrolyte solution. The SI unit of electrochemical equivalent is kilogram per coulomb (kg/C).
Table 1.
Electrochemical equivalents of substances
| Substance (anions) | K, 10–6 kg/Kl | Substance (cations) | K, 10–6 kg/Kl |
| Hydroxyl (OH–) | 0,177 | Aluminum (Al3+) | 0,0932 |
| Oxygen (O2–) | 0,0829 | Hydrogen (H+) | 0,1045 |
| Acid residue (SO42–) | 0,499 | Iron (Fe3+) | 0,193 |
| Sulfur (S2–) | 0,167 | Gold (Au3+) | 0,681 |
| Chlorine (Cl–) | 0,367 | Copper (Cu2+) | 0,329 |
| Sodium (Na+) | 0,238 | ||
| Nickel (Ni2+) | 0,30 | ||
| Silver (Ag+) | 1,11 | ||
| Mercury (Hg+) | 2,079 | ||
| Zinc (Zn2+) | 0,339 |
Faraday's second law:
- the electrochemical equivalent of a substance K
is directly proportional to its chemical equivalent:
\[K=C\cdot \dfrac{M}{Z} .\]
Here C
- proportionality coefficient, constant value, ratio $\dfrac{M}{Z} $ - chemical equivalent,
M
- molar mass of the substance,
Z
- valency of the substance.
This law is usually written in a different form, taking into account that $C = \dfrac{1}{F}$, where F
called Faraday's constant:
\[K=\frac{1}{F} \cdot \dfrac{M}{Z} .\]
- Faraday's constant F
is equal to the product of the elementary charge
e
and Avogadro's number
NA
:
F = e⋅NA
,
F
= 9.65⋅104 C/mol.
Faraday's laws played an important role in the history of the development of physics. They served as an impetus for putting forward the hypothesis about the existence of an elementary electric charge in nature and made it possible for the first time to determine its value.
See also
- Kikoin A.K. On the Faraday number and the specific charge of a charged particle // Quantum. - 1985. - No. 2. - P. 25-26
Application of electrolysis in technology
Electrolysis is widely used in technology.
Purification or refining of metals
. The process takes place in an electrolytic bath. The anode is the metal to be purified, the cathode is a thin plate of pure metal, and the electrolyte is a solution of a salt of a given metal, for example, when refining copper, a solution of copper sulfate. Contaminated metals may contain valuable impurities. Thus, copper often contains nickel and silver. In order for only pure metal to be released at the cathode, it must be taken into account that the release of each substance begins only at a certain potential difference between the electrodes, called the “decomposition potential”. With proper selection, pure copper is released from the copper sulfate solution at the cathode, and impurities precipitate or pass into solution.
Electrometallurgy
. Some metals, such as aluminum, are produced by electrolysis from molten ore. An iron box with a carbon floor serves as an electrolytic bath and at the same time a cathode, and carbon rods serve as an anode. The temperature of the ore (about 900 °C) is maintained by the current flowing through it. Molten aluminum falls to the bottom of the box, from where it is released through a special hole into molds for casting.
Galvanostegy
- an electrolytic method of coating metal products with a layer of noble or other metal (gold, platinum) that cannot be oxidized. For example, when nickeling an object, it itself serves as a cathode, and a piece of nickel serves as an anode. By passing electric current through the electrolytic bath for some time, the object is coated with a layer of nickel of the required thickness.
Electrotype
, or the electrolytic deposition of metal on the surface of an object to reproduce its shape, was invented in 1837 by the Russian scientist B. S. Jacobi, who proposed using electrolysis to obtain metal prints of relief objects (medals, coins, etc.). A wax cast is taken from the object or a convex image is cut out on a wooden board and made conductive by covering it with a layer of graphite. The cast or board is then dipped into the electrolyte as a cathode. The anode is a piece of metal used for deposition. This method is used to make, for example, printing clichés.
Heavy water (D2O) is produced electrolytically, in which the hydrogen atoms are replaced by atoms of its isotope, deuterium (D) with an atomic mass of 2.
See also
- Myakishev G.Ya. Physics: Electrodynamics //§3.6. Technical Application of Electrolysis
Introduction
A lot in our lives is connected with the electrical conductivity of solutions of salts in water (electrolytes). From the first beat of the heart (“living” electricity in the human body, which is 80% water) to cars on the street, players and mobile phones (an integral part of these devices are “batteries” - electrochemical batteries and various batteries - from lead-acid in cars to lithium polymer in the most expensive
mobile phones). In huge vats smoking with toxic fumes, aluminum is produced by electrolysis from bauxite melted at high temperatures - the “winged” metal for airplanes and cans for Fanta. Everything around – from the chrome-plated radiator grill of a foreign car to a silver-plated earring – has ever encountered a solution or molten salts, and, consequently, an electric current in liquids.
Literature
- Aksenovich L. A. Physics in secondary school: Theory. Tasks. Tests: Textbook. allowance for institutions providing general education. environment, education / L. A. Aksenovich, N. N. Rakina, K. S. Farino; Ed. K. S. Farino. - Mn.: Adukatsiya i vyakhavanne, 2004. - P. 282-287.
- Burov L.I., Strelchenya V.M. Physics from A to Z: for students, applicants, tutors. - Mn.: Paradox, 2000. - P. 228-232.
- Zhilko, V.V. Physics: textbook. allowance for 11th grade. general education school from Russian language training / V.V. Zhilko, A.V. Lavrinenko, L. G. Markovich. — Mn.: Nar. Asveta, 2002. - pp. 258-263.