Not to be confused with farad.
B Faraday's Constant
, symbolized
F
and sometimes stylized as ℱ, named after Michael Faraday.
In chemistry and physics, this constant represents the amount of electric charge per mole of electrons.[1] Has the current accepted value F
= 96485.33212… C mol−1.[2]
Since 1 mole of electrons = 6.02214076×1023 electrons (Avogadro's number), [3] Faraday's constant is equal to the elementary charge e
, the amount of electron charge multiplied by 1 mole: [4]
F
= 96485.3... C / (1 mol) = 96485.3... C / (6.022...×1023) = 1.60217663410×10−19 C =
e
One of the common ways to use Faraday's constant is electrolysis calculations. The amount of charge can be divided by the coulombs of Faraday's constant to find the chemical amount (in moles) of the element that has been oxidized.
F value
was first determined by weighing the amount of silver deposited in an electrochemical reaction in which a measured current was passed for a measured time, and by using Faraday's Law of Electrolysis.[5]
History of development and experiments of Faraday
Until the middle of the 19th century, it was believed that the electric and magnetic fields have no connection, and the nature of their existence is different. But M. Faraday was confident in the unified nature of these fields and their properties. The phenomenon of electromagnetic induction, discovered by him, subsequently became the foundation for the design of generators of all power plants. Thanks to this discovery, humanity's knowledge of electromagnetism has made great strides forward.
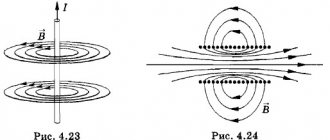
Faraday performed the following experiment: he closed a circuit in coil I and the magnetic field around it increased. Next, the induction lines of this magnetic field crossed coil II, in which an induced current arose.
Rice. 1. Scheme of Faraday's experiment
In fact, simultaneously with Faraday, but independently of him, another scientist, Joseph Henry, discovered this phenomenon. However, Faraday published his research earlier. Thus, the author of the law of electromagnetic induction was Michael Faraday.
No matter how many experiments Faraday conducted, one condition remained unchanged: for the formation of an induction current, it is important to change the magnetic flux penetrating a closed conducting circuit (coil).
Self-induction
Self-induction is the phenomenon of the occurrence of induced emf in a conductor as a result of a change in current in it.
When the current in the coil changes, the magnetic flux created by this current changes. A change in the magnetic flux passing through the coil should cause the appearance of an induced emf in the coil.
In accordance with Lenz's rule, the self-inductive emf prevents the current from increasing when the circuit is turned on and the current from decreasing when the circuit is turned off.
This leads to the fact that when a circuit is closed in which there is a current source with a constant EMF, the current strength is established after some time.
When the source is turned off, the current also does not stop instantly. The self-inductive emf that arises in this case may exceed the source emf.
The phenomenon of self-induction can be observed by assembling an electrical circuit from a coil with high inductance, a resistor, two identical incandescent lamps and a current source. The resistor must have the same electrical resistance as the coil wire.
Experience shows that when the circuit is closed, an electric lamp connected in series with a coil lights up somewhat later than a lamp connected in series with a resistor. The increase in current in the coil circuit during closure is prevented by the self-induction emf, which occurs when the magnetic flux in the coil increases.
When the power source is turned off, both lamps flash. In this case, the current in the circuit is maintained by the self-induction emf that occurs when the magnetic flux in the coil decreases.
Self-induction emf ( varepsilon_{is} ), arising in a coil with inductance ( L ), according to the law of electromagnetic induction is equal to:
The self-inductive emf is directly proportional to the inductance of the coil and the rate of change of current in the coil.
Experience with two coils
The experiment with two coils consisted of passing a current through one of them, and a galvanometer was connected to the other. At the moment the current began or ended passing through the first coil, the needle of the galvanometer connected to the second coil oscillated. This Faraday experiment showed that not only magnetism can be converted into electricity, but also electricity into magnetism. An alternating current passed through one of two coils located close to each other turned it into a magnet, inducing a current in the neighboring one. The characteristics of the magnetic field (polarity, intensity) depended on the strength of the current passed.
Modern transformers used in electronics and electrical engineering operate on the principle of interaction of coils with alternating current.
Inductance
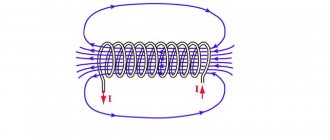
Passing along the circuit, the electric current contributes to the formation of a set of magnetic lines of force around it. According to the formula Ф = L×I, the flux Ф created by the magnet depends proportionally on the current strength I.
Thus, inductance L is understood as the coefficient of the ratio between the magnetic flux Ф and the current I flowing through the circuit. This value is calculated using the following formula:
L=F/I.
The unit of measurement for this physical quantity is Henry (H). 1 H is the inductance formed in a closed circuit in which the current changes by 1 Ampere, and the voltage in it is 1 Volt.
Second Law
Faraday, passing an electric current of the same strength through various electrolytes, noticed that the masses of substances on the electrodes were not the same. After weighing the released substances, Faraday concluded that the weight depended on the chemical nature of the substance. For example, for every gram of hydrogen released there were 107.9 g of silver, 31.8 g of copper, 29.35 g of nickel.
Based on the data obtained, Faraday derived the second law of electrolysis: for a certain amount of electricity, the mass of a chemical element formed on the electrode is directly proportional to the equivalent mass of the element. It is equal to the mass of one equivalent - the amount of substance that reacts or replaces 1 mole of hydrogen atoms in chemical reactions:
μeq = μ/z,
Where:
- μ – molar mass of the substance;
- z is the number of electrons per ion (valence number of ions).
To release one mole equivalent, the same amount of electricity is consumed - 96485 C/mol. This number is called the Faraday number and is symbolized by the letter F.
According to the second law, the electrochemical equivalent is directly proportional to the equivalent mass of the substance:
k = (1/F) μeq or k = (1/zF)μ.
Rice. 3. Faraday's second law.
Faraday's two laws can be reduced to a general formula: m = (q / F) ∙ (μ/z).
The phenomenon of electromagnetic induction
Electromagnetic induction is the phenomenon of the occurrence of current in a closed conductive circuit when the magnetic flux passing through it changes.
The phenomenon of electromagnetic induction was discovered by M. Faraday.
Faraday's experiments
- Two coils were wound on one non-conducting base: the turns of the first coil were located between the turns of the second. The turns of one coil were closed to a galvanometer, and the second was connected to a current source. When the key was closed and current flowed through the second coil, a current pulse arose in the first. When the switch was opened, a current pulse was also observed, but the current through the galvanometer flowed in the opposite direction.
- The first coil was connected to a current source, the second, connected to a galvanometer, moved relative to it. As the coil approached or moved away, the current was recorded.
- The coil is closed to the galvanometer, and the magnet moves - moves in (extends) - relative to the coil.
Experiments have shown that induced current occurs only when the magnetic induction lines change. The direction of the current will be different when the number of lines increases and when they decrease.
The strength of the induction current depends on the rate of change of the magnetic flux. The field itself may change, or the circuit may move in a non-uniform magnetic field.
Explanations of the occurrence of induction current
Current in a circuit can exist when external forces act on free charges. The work done by these forces to move a single positive charge along a closed loop is equal to the emf. This means that when the number of magnetic lines through the surface limited by the contour changes, an emf appears in it, which is called the induced emf.
Electrons in a stationary conductor can only be driven by an electric field. This electric field is generated by a time-varying magnetic field. It is called the vortex electric field. The concept of a vortex electric field was introduced into physics by the great English physicist J. Maxwell in 1861.
Properties of the vortex electric field:
- source – alternating magnetic field;
- detected by the effect on the charge;
- is not potential;
- the field lines are closed.
The work of this field when moving a single positive charge along a closed circuit is equal to the induced emf in a stationary conductor.
The essence of the electrolysis process
Electrolysis refers to the processes of redox reactions that occur under the forced influence of electric current. To perform this, a special container with an electrolytic solution is used, into which metal pins connected to an external power source are immersed.
The electrode connected to the negative pole of the current source is considered the cathode. It is at this point that the electrolyte particles are reduced. The other electrode is connected to the positive pole and is called the anode. In this area, the electrode substance or electrolyte particles are oxidized. Chemical reactions in this area occur differently, depending on the anode material and the composition of the electrolytic solution. Therefore, as chemistry states, electrodes in relation to the electrolyte can be inert or soluble.
The inert category includes anodes made from a material that does not oxidize during electrolysis. Examples include graphite or platinum electrodes. Almost all other types of metal anodes that are subject to oxidation during an electrolytic reaction are soluble.
Electrolytes most often serve as various types of solutions or melts, within which there is a chaotic movement of charged particles - ions. When they are exposed to electric current, they begin to move in a certain direction: cations - towards the cathode, anions - towards the anode. When they get on the electrodes, they lose their charges and settle on them.
First generator
In 1831, the scientist, in order to clearly demonstrate the process of converting mechanical energy into electrical energy, built a Faraday generator. This device had no practical significance, but clearly showed the magic of the creation of electric current.
The Faraday disk was a device resembling a primitive generator. In this design, the magnetic field was directed along the axis of rotation, and the circuit was left motionless. Observers were surprised by the fact that the rotation of the magnet together with the disk led to the appearance of an electromotive force in the stationary circuit. This phenomenon was called Faraday's paradox. This contradiction was resolved after the scientist’s death, when the electron was discovered, which behaves both as a charge and as a particle.