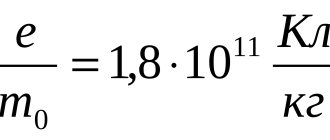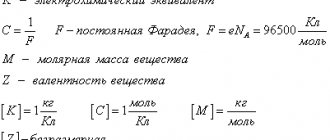4.7
Average rating: 4.7
Total ratings received: 61.
4.7
Average rating: 4.7
Total ratings received: 61.
Electrolytes are substances whose solutions or melts are capable of conducting electricity. The movement of electric current in electrolytes is called electrolysis.
Electricity transmission
Electric current is the ordered movement of charged particles. The charge carriers of electric current in electrolytes are ions. They are formed as a result of the disintegration (electrolytic dissociation) of substance molecules under the action of water molecules in solution or upon heating and the formation of a melt.
The splitting of molecules occurs due to the breaking of polar covalent or ionic bonds. The intensity of dissociation depends on the temperature and concentration of the solution. The nature of the electrolyte also influences the degree of dissociation. In this regard, the following are highlighted:
- weak electrolytes , which disintegrate partially or not at all;
- strong electrolytes that quickly break down into ions.
Weak electrolytes include most organic substances, weak acids, poorly soluble salts and insoluble bases. Strong acids, alkalis, and salts are considered strong electrolytes.
Rice. 1. The process of electrolytic dissociation.
The ions formed as a result of dissociation are divided into two types:
- cations are positively charged particles;
- Anions are negatively charged particles.
The conductor of electric current in electrolytes is the electrode. It can be an anode or cathode. The anode is connected to the positive pole of the current source, the cathode is connected to the negative pole. The anode oxidizes the substances in the electrolyte, while the cathode reduces them.
Rice. 2. Electrodes.
If two electrodes are placed in an electrolyte solution - the cathode and the anode - and an electric current is turned on, the ions will begin to move under the influence of the electric field. Cations will rush to the cathode, anions to the anode. Having reached the electrodes, the ions are neutralized, turn into neutral atoms and settle.
The process of decomposition of a substance into its constituent parts, which are deposited on electrodes, is called electrolysis.
Electric current transmission
Electric current represents the ordered movement of free charges. To find out how electric current is conducted in solutions, you need to understand which particles are its carriers. In solids, current is created by electrons. The carriers of electric current in electrolytes are ions. These particles are formed as a result of the process of decay (electrical dissociation) of the molecules of a substance under the influence of water in solutions or upon heating and the subsequent appearance of a melt.
Molecules of substances disintegrate due to the rupture of ionic or polar covalent bonds. The number of charge carriers in the electrolyte is determined by concentration and temperature. In addition, the degree of decomposition of molecules depends on the nature of the electrolyte. As a result, they are divided into two groups:
- weak - not subject to decay or this process proceeds extremely slowly;
- strong - in such electrolytes, rapid splitting of molecules into ions is observed.
The first group includes most of the organic substances - insoluble bases, weak acids and poorly soluble salts. Strong electrolytes are alkalis, strong acids and highly soluble salts.
Faraday's law
The electrolysis process was experimentally studied by the English physicist and chemist Michael Faraday in 1833. He formulated a law according to which the mass of the substance released on the electrode is directly proportional to the charge passing through the electrolyte. This law became established in science as Faraday's first law.
Rice. 3. Michael Faraday.
m = kQ = kIt,
Where:
- m is the mass of the substance;
- Q – charge;
- k – electrochemical equivalent;
- I – current strength;
- t is the duration of the current.
According to Faraday's second law, the mass of the substance released onto the electrodes is directly proportional to the ratio of molar mass to valence and is equal to the electrochemical equivalent.
m = k = M/z,
Where:
- m is the mass of the released substance;
- k – electrochemical equivalent;
- M – molar mass;
- z is the valency of the substance.
Electrolysis is used in alkaline and acid batteries. Electrolysis can be used to protect a product with a metal coating.
Ionic conductivity
In metals, as you remember, there is only one type of free charges - free electrons. In electrolytes the situation is different: free charges of two types arise here.
1. Positive ions formed from metal or hydrogen atoms.
2. Negative ions - atomic or molecular acidic residues (for example, or), as well as a hydroxyl group.
The second difference from metals is that carriers of free charges in an electrolyte can have a charge equal in magnitude to both the elementary charge and an integer number of elementary charges. Here is the valency of an atom or group of atoms; for example, when dissolving copper sulfate we have .
If there is no external electric field, then the free charges of the electrolyte undergo only chaotic thermal motion along with the surrounding molecules. But when an external field is applied, positive and negative ions begin to move in an orderly manner.
Place two electrodes in a vessel with electrolyte; We connect one of the electrodes to the positive terminal of the current source, and the other to the negative terminal (Fig. 5). When it comes to the passage of current through electrolytes, the positive electrode is called the anode
, and the negative one is
the cathode
(There is folk wisdom for remembering the signs of the anode and cathode:
Andrey is a positive guy, Katka is a negative girl
;-)).
Rice. 5. Ionic conductivity of the electrolyte
In the electric field that arises between the electrodes, positive ions of the electrolyte rush to the “minus” of the cathode, and negative ions to the “plus” of the anode. Thus, the electric current in the electrolyte is formed as a result of the counter-movement of ions: positive - to the cathode, negative - to the anode
.
Therefore, the conductivity of electrolytes is called ionic
(in contrast to the electronic conductivity of metals).
There is a lack of electrons at the positive anode. Negative ions, having reached the anode, give it their extra electrons; these electrons are sent along the circuit to the “plus” of the source.
On the contrary, there is an excess of electrons at the negative cathode. Positive ions, arriving at the cathode, take electrons from it, and this number of lost electrons is immediately replenished by their delivery to the cathode from the “minus” source.
Thus, in that part of the circuit that consists of a current source and metal conductors, electron circulation occurs along the “anode source cathode” route.
The circuit is closed by the electrolyte, where the electric current is provided by the two-way movement of ions.
Electric current in electrolytes
In liquid conductors ( electrolytes ), there is a continuous spontaneous disintegration of molecules into their component parts. This process is sometimes called the molecular dissociation . For example, a molecule of copper sulfate CuS04 breaks down into a positive copper ion Cu and a negative ion S04 (the so-called acid residue). In an electrolyte, random thermal movement of ions and molecules occurs. The ions combine, form molecules, break up again, etc. In general, the solution is electrically neutral, since the number of positive and negative ions in it is the same.
Let's do the following experiment. We lower two copper plates (electrodes) into a glass vessel with a solution of copper sulfate (CuS04) and connect them to a source of electrical energy (Fig. 1). The plate connected to the positive pole of the source is called the anode (this plate has a positive potential), and the other connected to the negative pole is called the cathode (this plate has a negative potential).
Figure 1. Passage of electric current through a solution of copper sulfate.
As soon as we connect the plates to a source of electrical energy, an electric field is formed between them. Electrical forces will begin to act on the positive and negative ions in the solution.
It is obvious that negative ions ( SO4 ) will go to the anode, and positive ( Cu ) to the cathode, i.e., ordered movement of ions will begin in the electrolyte. This ordered movement of ions in an electrolyte under the influence of electric field forces is called ionic electric current . An ionic electric current exists as long as there is a potential difference between the electrodes.
We see a significant difference between the electric current in an electrolyte and the electric current in a metal conductor: the first is created by the movement of ions, and the second by free electrons.
What will happen in the electrolyte after positive ions approach the cathode and negative ions approach the anode?
Positive ions (Cu) attach the missing electrons and turn into pure copper molecules. Copper molecules in the form of a thin layer are deposited on the cathode. Negative ions donate excess electrons to the anode and enter into a chemical reaction with the anode material, i.e., copper, forming molecules of copper sulfate (CuSO4). These molecules enter the electrolyte.
Thus, in the electrolyte, when an electric current passes, the following process occurs: the cathode is covered with a layer of copper released from the electrolyte, and the anode dissolves and replenishes the loss of copper in the electrolyte.
Definition: The process of passage of ionic electric current in an electrolyte, accompanied by chemical transformations of the substance and its release, is called electrolysis
The honor of discovering the phenomenon of electrolysis belongs to the Russian academician B. S. Jacobi. Currently, the phenomenon of electrolysis is widely used in industry (cleaning metals, making copies of various objects, nickel plating, gilding, silvering, etc.).
DID YOU LIKE THE ARTICLE? SHARE WITH YOUR FRIENDS ON SOCIAL NETWORKS!
Related materials:
- Current flow
- Electric current in metal conductors
- Electromotive force (EMF) of the energy source
- Direction and magnitude of electric current. Amount of electricity
- Electrical resistance of a conductor. Electrical conductivity
- Bias current in dielectric
- Electric current in semiconductors
- Electric current in gases
Add a comment
Electrical dissociation
This is a fundamental process for the appearance of electric current in solutions, so it needs to be considered in more detail. All ions formed during the decay of molecules can be divided into 2 types:
- Anions. They have a negative charge.
- Cations. They have a positive charge.
- the angle between the central lines of the atoms is approximately 104.5 degrees;
- electrons are shifted towards oxygen.
Most of the properties of water are due to the polarity of the molecules of the substance. In other words, from the point of view of electrical engineering they are dipoles. Here we should recall the definition of a dipole - a system of two particles located close to each other. Moreover, their charges are opposite in sign, but identical in magnitude. The polarity property of H2O is explained by the geometric structure of the molecules of the substance:
Being dipoles, water molecules are able to create an electric field around themselves, which affects not only them, but also the particles of the dissolved substance.
To establish the nature of the process of decomposition of molecules into ions, we should consider a solution of table salt. There is only 1 electron in the outer orbit of the sodium atom. His connection with the atom is weak, so he is able to quickly leave his place. The chlorine atom already has 7 electrons in its outer orbit and is one particle short of the complete set. Due to this, during the formation of a NaCl crystal, the outer electron of sodium is added to the chlorine atom. As a result, a dipole is formed.
The interaction of two types of dipoles contributes to the activation of the dissolution process. If you place 2 electrodes in an electrolyte solution - the cathode (negative) and the anode (positive), then free ions will rush towards them. In this case, the direction of their movement follows specific rules:
- cations will go to the cathode;
- anions begin to move towards the anode.
As soon as the electric current carriers reach the electrodes, they lose their charge, turning neutral, and settle on the surface of the electrodes.