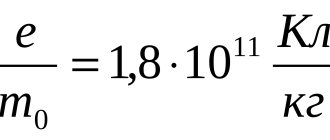Electric current in liquids
As you know, chemically pure (distilled) water is a poor conductor. However, when various substances (acids, alkalis, salts, etc.) are dissolved in water, the solution becomes a conductor due to the breakdown of the molecules of the substance into ions. This phenomenon is called electrolytic dissociation
, and the solution itself
is an electrolyte
capable of conducting current.
Unlike metals and gases, the passage of current through an electrolyte is accompanied by chemical reactions at the electrodes, which leads to the release of chemical elements that make up the electrolyte on them.
Faraday's first law: the mass of a substance released on any of the electrodes is directly proportional to the charge passing through the electrolyte
The electrochemical equivalent of a substance is a tabular value.
Faraday's second law:
The flow of current in liquids is accompanied by the release of heat. In this case, the Joule-Lenz law is fulfilled.
Online electrical magazine
Electron current in liquids
In an iron conductor, an electron current appears through the directed movement of free electrons, and in all this, no changes in the substance from which the conductor is made occur.
Such conductors, in which the passage of electronic current is not accompanied by chemical changes in their substance, are called conductors of the first kind. These include all metals, coal and a number of other substances.
But there are also conductors of electronic current in nature in which chemical phenomena occur during the passage of current. These conductors are called conductors of the second kind. These include mainly different mixtures of acids, salts and alkalis in water.
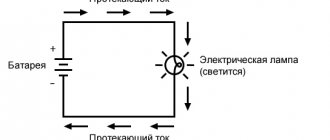
If you pour water into a glass vessel and add a few drops of sulfuric acid (or some other acid or alkali), and then take two iron plates and connect conductors to them, lowering these plates into the vessel, and connect a current source to the other ends of the conductors through the switch and ammeter, then gas will be released from the solution, and it will last continuously as long as the circuit is closed because acidified water is indeed a conductor. In addition, the plates will begin to become covered with gas bubbles. Then these bubbles will break away from the plates and come out.
When an electron current passes through the solution, chemical changes occur, resulting in the release of gas.
Conductors of the second kind are called electrolytes, and the phenomenon that occurs in an electrolyte when an electron current passes through it is called electrolysis.
Iron plates immersed in an electrolyte are called electrodes; one of them, connected to the positive pole of the current source, is called the anode, and the other, connected to the negative pole, is called the cathode.
What determines the passage of electron current in a watery conductor? It turns out that in such mixtures (electrolytes), acid (alkali, salt) molecules under the influence of a solvent (in this case water) split into two component parts, with one particle of the molecule having a positive electronic charge and the other negative.
Molecular particles that have an electronic charge are called ions. When an acid, salt or alkali is dissolved in water, a huge number of both positive and negatively charged ions appear in the solution.
Now it should become clear why an electron current passed through the solution, because a potential difference was created between the electrodes connected to the current source, in other words, one of them turned out to be positively charged, and the other negatively. Under the influence of this potential difference, positive ions began to mix towards the negative electrode - the cathode, and negative ions - towards the anode.
Thus, the chaotic movement of ions became an ordered counter movement of negatively charged ions in one direction and positive ones in the other. This process of charge transfer constitutes the flow of electron current through the electrolyte and occurs as long as there is a potential difference across the electrodes. With the disappearance of the potential difference, the current through the electrolyte stops, the ordered movement of ions is disrupted, and chaotic movement begins again.
As an example, let us consider the phenomenon of electrolysis when passing an electron current through a solution of copper sulfate CuSO4 with copper electrodes lowered into it.
The phenomenon of electrolysis when current passes through a solution of copper sulfate: C - vessel with electrolyte, B - current source, C - switch
There will also be a counter movement of ions to the electrodes. The positive ion will be the copper ion (Cu), and the negative ion will be the acid residue ion (SO4). Copper ions in contact with the cathode will be discharged (attaching the missing electrons to themselves), i.e., converted into neutral molecules of pure copper, and deposited on the cathode in the form of a thin (molecular) layer.
Negative ions, having reached the anode, are also discharged (they give up extra electrons). But at the same time, they enter into a chemical reaction with the copper of the anode, as a result of which a molecule of copper Cu is added to the acidic residue SO4 and a molecule of copper sulfate CuSO4 appears, which is returned back to the electrolyte.
Because this chemical process takes a long time, copper is deposited on the cathode, released from the electrolyte. In this case, the electrolyte, instead of the copper molecules that went to the cathode, receives new copper molecules due to the dissolution of the second electrode - the anode.
The same process occurs if zinc electrodes are taken instead of copper ones, and a solution of zinc sulfate ZnSO4 serves as the electrolyte. Zinc will also be transferred from the anode to the cathode.
Thus, the difference between the electron current in metals and liquid conductors is that in metals the charge carriers are only free electrons, i.e., negative charges, while in electrolytes electricity is carried by differently charged particles of matter - ions moving in opposite directions . Therefore, they say that electrolytes have ionic conductivity.
The phenomenon of electrolysis was discovered in 1837 by B. S. Jacobi, who created countless experiments to study and improve chemical current sources. Jacobi found that one of the electrodes placed in a solution of copper sulfate became coated with copper when an electron current passed through it.
This phenomenon, called galvanoplasty, currently has a very wide practical application. One example of this is the coating of iron objects with a thin layer of other metals, i.e. nickel plating, gilding, silvering, etc.
Electron current in gases
Gases (including air) do not conduct electron current under ordinary conditions. For example, naked wires of overhead lines, being suspended parallel to each other, are isolated from one another by a layer of air.
But under the influence of high temperatures, large potential differences and other circumstances, gases, like watery conductors, are ionized, that is, particles of gas molecules appear in them in large quantities, which, being carriers of electricity, facilitate the passage of electron current through the gas.
But at the same time, the ionization of a gas differs from the ionization of a watery conductor. If in water a molecule disintegrates into two charged parts, then in gases, under the influence of ionization, electrons are always separated from each molecule and an ion remains in the form of a positively charged part of the molecule.
As soon as the ionization of the gas is completed, it will cease to be conductive, while the liquid always remains a conductor of electron current. As follows, the conductivity of gas is a temporary phenomenon, depending on external circumstances.
But there is another type of discharge, called an arc discharge or simply an electronic arc. The phenomenon of the electron arc was discovered in the early 19th century by the first Russian electrical engineer V.V. Petrov.
V.V. Petrov, through countless experiments, found that between two charcoals connected to a current source, a continuous electronic discharge appears through the air, accompanied by a bright light. In his own writings, V.V. Petrov wrote that with all this, “the black peace can be quite brightly illuminated.” This is how electronic light was obtained for the first time, which was actually used by another Russian electrical engineer Pavel Nikolaevich Yablochkov.
The Yablochkov Candle, whose operation is based on the use of an electronic arc, made a real revolution in electrical engineering in those days.
The arc discharge is used as a light source today, for example, in spotlights and projection devices. The high temperature of the arc discharge makes it possible to use it for the construction of an arc furnace. Currently, arc furnaces, powered by very high current, are used in a number of areas of industry: for the smelting of steel, cast iron, ferroalloys, bronze, etc. And in 1882, N.N. Benardos used an arc discharge for the first time for cutting and welding metal.
In gas-light tubes, fluorescent lamps, voltage stabilizers, the so-called glowing gas discharge is used to produce electric and ion beams.
The spark discharge is used to measure huge potential differences using a ball gap, the electrodes of which are two iron balls with a polished surface. The balls are moved apart and a measured potential difference is applied to them. Then the balls are brought closer together until a spark jumps between them. Knowing the diameter of the balls, the distance between them, pressure, temperature and humidity, find the potential difference between the balls using special tables. This method can determine, with an accuracy of a few percent, potential differences of the order of 10 thousand volts.
That's all for now. Well, if you want to find out more, I recommend paying attention to Misha Vanyushin’s disk:
“About electricity for beginners in video format on DVD”
Electric current in metals
When current passes, the metals heat up. As a result, the ions of the crystal lattice begin to vibrate with greater amplitude near the equilibrium positions. As a result, the flow of electrons more often collides with the crystal lattice, and consequently the resistance to their movement increases. As the temperature increases, the resistance of the conductor increases.
Each substance is characterized by its own temperature coefficient of resistance - a tabular value. There are special alloys whose resistance practically does not change when heated, for example manganin and constantan.
The phenomenon of superconductivity.
At temperatures close to absolute zero (-2730C), the resistivity of the conductor abruptly drops to zero. Superconductivity is a microscopic quantum effect.
ELECTRICAL PROPERTIES OF SOLIDS
All materials conduct electric current to one degree or another, i.e. have electrical conductivity. Based on this criterion, materials are divided into conductors, semiconductors, and dielectrics.
The ability and ability of a material to conduct electric current is mainly determined by: the type of chemical bond; bandgap width; the type of free charge carriers, their concentration and mobility.
The main parameters characterizing electrical properties are: electrical conductivity g(Ohm-1 m-1); electrical resistivity ρ (Ohm m); temperature coefficient of electrical resistivity aρ, or TCR (K-1).
Electrical conductivity
g connects the current density
j
(A/m2) and the electric field strength
E
(V/m) causing this current by the relation
j
= g
E
(differential form of Ohm’s law).
Electrical resistivity -
the reciprocal of electrical conductivity: ρ = 1/g.
For a body with a constant cross section S,
resistance
R
and length
l
ρ is determined by the formula
ρ = RS/l.
According to the theory of electrical conductivity, g can be expressed by the following formula:
g = q2nl/(m v
),
where q
and
m are,
respectively, the charge and mass of the charge carrier (electron in conductors, electron and hole in semiconductors, ions in dielectrics);
v
and l are the speed and free path of the charge carrier;
n is
the concentration of charge carriers, i.e. their number per unit volume.
The change in electrical conductivity, and therefore the electrical resistivity, in real materials is associated with a change in the concentration and free path of charge carriers.
Under the influence of an electric field, charge carriers acquire acceleration, and their speed is proportional to the field strength:
v
=
uE,
where and
(m2 / V s) - mobility of charge carriers - the ratio of the speed of their directional movement caused by an electric field to the strength of this field. It is defined by the expression
And
=
ql/(mv),
whence
g = qnu.
The magnitude of electrical conductivity strongly depends on the scattering of carriers by imperfections of the crystal lattice—structural defects and phonons. As a result of scattering, the mean free path, speed and mobility of charge carriers decrease.
Electrons in an isolated atom have strictly defined discrete energy values. In a solid body, due to the approach of atoms and the strong interaction of electrons and nuclei, the energy levels of atoms are split and combined into energy zones
(Fig. 4.1).
The energy band formed by splitting the levels of valence electrons is called the valence band (Ev).
The next allowed energy band is
the conduction band (Ec).
Between them there is
a band gap (Eg).
If an electron receives energy exceeding the band gap, then it moves from the valence band to the conduction band and participates in electrical conductivity.
In accordance with band theory, solids are divided into conductors, semiconductors and dielectrics.
Conductors
- materials in which the valence band and conduction band overlap or are adjacent to each other, so the electrons in the metal are free, i.e.
can move from the valence band to the conduction band when a small electric field is applied. Atoms in metals are connected to each other by metallic bonds.
Valence electrons have high mobility and, due to the overlap of
Ev
and
Ec
easily move in the lattice of a metal crystal.
Electronic type is observed in metals
electrical conductivity. In this case, the electrons accelerated by the field transfer only charge. Mass transfer, as, for example, in materials with ionic conductivity, does not occur.
Rice. 4.1. Energy bands in a solid
The range of values of ρ for metal conductors occupies three orders of magnitude: from 1.58·10-8 Ohm·m for silver to 1000·10-8 Ohm·m for alloys of the Fe-Cr-A1 system.
Semiconductors
in terms of electrical properties, they occupy an intermediate position between conductors and dielectrics: their electrical resistivity is 10-6 -109 Ohm m, the band gap is from 0.05 to 2.5-3 eV (thermal motion energy at room temperature
kT
~ 0, 03 eV).
Atoms in semiconductors can be bonded by both nonpolar and polar covalent bonds, as well as ionic bonds; type of electrical conductivity - electron-hole.
Just like dielectrics, semiconductors have a negative temperature coefficient of resistance (TCR) aρ, i.e. With increasing temperature, the ρ of semiconductors decreases, while the ρ of metals increases.
An important feature of semiconductors is the high sensitivity of electrical resistivity not only to thermal, but also to other external influences (electromagnetic fields, radiation, pressure, etc.). This is due to the type of chemical bond between atoms in the crystal lattice of the semiconductor, as well as the presence of impurities and other defects, even insignificant concentrations of which significantly affect the concentration of free charge carriers and, consequently, the electrical properties of the material.
In industry, semiconductors are used that have both electron and hole
types of electrical conductivity.
In dielectrics
The band gap exceeds 3 eV, and the electrical resistivity is 109–1016 Ohm m.
Just as in semiconductors, dielectrics can have a covalent type of bond. A feature of the electrical conductivity of solid dielectrics is, in most cases, its ionic nature. Since Eg >> kT,
only a very small number of electrons can be detached from their atoms under the influence of thermal energy, and their contribution to electrical conductivity is negligible.
Ionic electrical conductivity
can be caused by the movement of both impurity ions and ions of the dielectric itself.
It should be noted that the electronic type of conductivity can be noticeable if a large number of donor and acceptor levels, respectively, are formed in the band gap near the bottom of the conduction band and the top of the valence band. The appearance of such levels can be caused by the presence of impurities and crystal lattice defects.
Electronic conductivity, caused by the presence of free electrons, manifests itself in strong electric fields and leads to insulation breakdown. With electronic conductivity, substance transfer does not occur, while with ionic conductivity this phenomenon is observed.
All materials conduct electric current to one degree or another, i.e. have electrical conductivity. Based on this criterion, materials are divided into conductors, semiconductors, and dielectrics.
The ability and ability of a material to conduct electric current is mainly determined by: the type of chemical bond; bandgap width; the type of free charge carriers, their concentration and mobility.
The main parameters characterizing electrical properties are: electrical conductivity g(Ohm-1 m-1); electrical resistivity ρ (Ohm m); temperature coefficient of electrical resistivity aρ, or TCR (K-1).
Electrical conductivity
g connects the current density
j
(A/m2) and the electric field strength
E
(V/m) causing this current by the relation
j
= g
E
(differential form of Ohm’s law).
Electrical resistivity -
the reciprocal of electrical conductivity: ρ = 1/g.
For a body with a constant cross section S,
resistance
R
and length
l
ρ is determined by the formula
ρ = RS/l.
According to the theory of electrical conductivity, g can be expressed by the following formula:
g = q2nl/(m v
),
where q
and
m are,
respectively, the charge and mass of the charge carrier (electron in conductors, electron and hole in semiconductors, ions in dielectrics);
v
and l are the speed and free path of the charge carrier;
n is
the concentration of charge carriers, i.e. their number per unit volume.
The change in electrical conductivity, and therefore the electrical resistivity, in real materials is associated with a change in the concentration and free path of charge carriers.
Under the influence of an electric field, charge carriers acquire acceleration, and their speed is proportional to the field strength:
v
=
uE,
where and
(m2 / V s) - mobility of charge carriers - the ratio of the speed of their directional movement caused by an electric field to the strength of this field. It is defined by the expression
And
=
ql/(mv),
whence
g = qnu.
The magnitude of electrical conductivity strongly depends on the scattering of carriers by imperfections of the crystal lattice—structural defects and phonons. As a result of scattering, the mean free path, speed and mobility of charge carriers decrease.
Electrons in an isolated atom have strictly defined discrete energy values. In a solid body, due to the approach of atoms and the strong interaction of electrons and nuclei, the energy levels of atoms are split and combined into energy zones
(Fig. 4.1).
The energy band formed by splitting the levels of valence electrons is called the valence band (Ev).
The next allowed energy band is
the conduction band (Ec).
Between them there is
a band gap (Eg).
If an electron receives energy exceeding the band gap, then it moves from the valence band to the conduction band and participates in electrical conductivity.
In accordance with band theory, solids are divided into conductors, semiconductors and dielectrics.
Conductors
- materials in which the valence band and conduction band overlap or are adjacent to each other, so the electrons in the metal are free, i.e.
can move from the valence band to the conduction band when a small electric field is applied. Atoms in metals are connected to each other by metallic bonds.
Valence electrons have high mobility and, due to the overlap of
Ev
and
Ec
easily move in the lattice of a metal crystal.
Electronic type is observed in metals
electrical conductivity. In this case, the electrons accelerated by the field transfer only charge. Mass transfer, as, for example, in materials with ionic conductivity, does not occur.
Rice. 4.1. Energy bands in a solid
The range of values of ρ for metal conductors occupies three orders of magnitude: from 1.58·10-8 Ohm·m for silver to 1000·10-8 Ohm·m for alloys of the Fe-Cr-A1 system.
Semiconductors
in terms of electrical properties, they occupy an intermediate position between conductors and dielectrics: their electrical resistivity is 10-6 -109 Ohm m, the band gap is from 0.05 to 2.5-3 eV (thermal motion energy at room temperature
kT
~ 0, 03 eV).
Atoms in semiconductors can be bonded by both nonpolar and polar covalent bonds, as well as ionic bonds; type of electrical conductivity - electron-hole.
Just like dielectrics, semiconductors have a negative temperature coefficient of resistance (TCR) aρ, i.e. With increasing temperature, the ρ of semiconductors decreases, while the ρ of metals increases.
An important feature of semiconductors is the high sensitivity of electrical resistivity not only to thermal, but also to other external influences (electromagnetic fields, radiation, pressure, etc.). This is due to the type of chemical bond between atoms in the crystal lattice of the semiconductor, as well as the presence of impurities and other defects, even insignificant concentrations of which significantly affect the concentration of free charge carriers and, consequently, the electrical properties of the material.
In industry, semiconductors are used that have both electron and hole
types of electrical conductivity.
In dielectrics
The band gap exceeds 3 eV, and the electrical resistivity is 109–1016 Ohm m.
Just as in semiconductors, dielectrics can have a covalent type of bond. A feature of the electrical conductivity of solid dielectrics is, in most cases, its ionic nature. Since Eg >> kT,
only a very small number of electrons can be detached from their atoms under the influence of thermal energy, and their contribution to electrical conductivity is negligible.
Ionic electrical conductivity
can be caused by the movement of both impurity ions and ions of the dielectric itself.
It should be noted that the electronic type of conductivity can be noticeable if a large number of donor and acceptor levels, respectively, are formed in the band gap near the bottom of the conduction band and the top of the valence band. The appearance of such levels can be caused by the presence of impurities and crystal lattice defects.
Electronic conductivity, caused by the presence of free electrons, manifests itself in strong electric fields and leads to insulation breakdown. With electronic conductivity, substance transfer does not occur, while with ionic conductivity this phenomenon is observed.
Electric current in gases
Gases in their natural state do not conduct electricity (they are dielectrics), since they consist of electrically neutral atoms and molecules. An ionized gas containing electrons, positive and negative ions can become a conductor.
Ionization can occur under the influence of high temperatures, various radiations (ultraviolet, x-rays, radioactive), cosmic rays, and collisions of particles with each other.
The ionized state of the gas is called plasma
. On the scale of the Universe, plasma is the most common state of matter. The Sun, stars, and the upper layers of the atmosphere are made of it.
The passage of electric current through a gas is called a gas discharge
.
A glow discharge flows in the “advertising” neon tube
. The glowing gas is “living plasma.”
An arc discharge
a spark discharge
in lightning. Here the electric field strength reaches the breakdown value. The current strength is about 10 MA!
A corona discharge
Basic notes on physics. Electric current in various environments. (Grade 10)
Basic notes for a physics lesson in 10th grade.
Electric current in various environments.
Electric current in metals.
Electric current in metals is the ordered movement of electrons.
Wire is wound onto a coil, the ends of which are soldered to discs isolated from each other. A galvanometer is attached to the ends of the disks using sliding contacts and the coil is brought into rapid motion and then abruptly stopped. After stopping, the free charged particles move for some time relative to the conductor by inertia, and a current arises in the coil. The current exists for a short time, because the movement of particles is prevented by the resistance of the conductor.
The charge transferred in this case is proportional to the ratio of the charge of the particles to their mass.
Different substances have different resistivities. As temperature changes, the resistance of the conductor changes.
Resistance increases, temperature increases, current decreases.
Conductor resistance taking into account its temperature:
Submits to Ohm's law for a section of a circuit.
Electric current in semiconductors.
A semiconductor is a substance whose resistivity decreases sharply with increasing temperature.
At temperatures close to zero, the resistivity is very high. As the temperature increases, the resistivity decreases and behaves like a dielectric.
When silicon is heated, the kinetic energy of the particles increases and individual bonds break. Some electrons are released and "holes" are formed.
Impurity conductivity in semiconductors.
The conductivity of conductors in the presence of impurities is called impurity conductivity. There are two types of impurity conductivity – electronic and hole.
If the impurity has a valence greater than the pure conductor, then free electrons appear.
The conductivity is electronic, the impurity in this case is donor, the semiconductor is an n-type semiconductor.
If the impurity has a valence lower than that of the pure semiconductor, then bond breaks—holes—appear.
The conductivity is hole, the impurity is acceptor, the semiconductor is a p-type semiconductor.
Most semiconductor devices use p- and n-type semiconductors.
The property of p and n junctions is used to rectify alternating electric current in semiconductor diodes.
Electric current in electrolytes.
When electrolytes dissolve under the influence of the electric field of polar water molecules, the electrolyte molecules disintegrate into ions.
If a vessel with an electrolyte solution is connected to an electrical circuit, then negative ions will begin to move to the positive electrode - the anode, and positive ions to the negative cathode.
Electric current in electrolytes is the ordered movement of positive and negative ions that are formed as a result of electrolytic dissociation.
Electrolysis is the process of releasing a pure substance at an electrode as a result of an oxidation reaction.
The mass of the released substance is determined by Faraday's law:
Electric current in a vacuum.
Current in a vacuum cannot exist independently, because vacuum is a dielectric. In this case, the current can be created using thermionic emission.
Thermionic emission is a phenomenon in which electrons escape from metals when heated. Such electrons are called thermionic electrons.
Electric current in gases.
Electric current in gases is the ordered movement of positive and negative ions and electrons that are formed as a result of the action of an ionizer.
The process of electric current flowing through a gas is called a gas discharge.
Electric current in a vacuum
Is it possible for electric current to propagate in a vacuum (from the Latin vacuum - emptiness)? Since there are no free charge carriers in a vacuum, it is an ideal dielectric. The appearance of ions would lead to the disappearance of the vacuum and the production of ionized gas. But the appearance of free electrons will ensure the flow of current through the vacuum. How to get free electrons in a vacuum? Using the phenomenon of thermionic emission
— emission of electrons by a substance when heated.
Vacuum diode, triode, cathode ray tube (in old TVs) are devices whose operation is based on the phenomenon of thermionic emission. The basic principle of operation: the presence of a refractory material through which current flows - the cathode
, a cold electrode that collects thermionic electrons -
anode
.
General conclusions
Thus, considering the topic of how electric current propagates in different media, we can note: in gases, ordered movement begins under the influence of an electric field.
Electric current in various environments - solutions and melts of electrolytes. Many electrolytes in their normal state are dielectrics. But after dissolving them in water, these substances become conductors. This process is called electrolytic dissociation. Electric current flows in different media and solution under the influence of an external electric field. In this case, some ions move towards the cathode, while others move towards the anode.