LIQUID DIELECTRICS
Liquid dielectrics are low-molecular substances of organic origin, which can be polar or non-polar. Their electrical properties largely depend on the structure of the molecules and the presence of impurities. Impurities are formed during the oxidation and decomposition of hydrocarbon fractions, during the absorption of water and the ingress of particles of fibrous materials.
Liquid dielectrics are characterized by dielectric constant ε, electrical conductivity, dielectric losses (dielectric loss tangent tgδ), electrical strength E.
For polar liquids (Sovol, hexol, ethylene glycol), the dielectric constant ε is determined simultaneously by electronic and dipole polarizations. For example, for hexol ε = 3, for ethylene glycol ε = 40.
In non-polar liquids, the dielectric constant is determined mainly only by electronic polarization, does not depend on frequency and decreases with increasing temperature, approaching unity.
This phenomenon is explained by a decrease in the number of molecules per unit volume. Non-polar liquids have ε less than polar liquids. For example, for carbon tetrachloride ε = 2.163, for toluene ε = 2.294.
The electrical conductivity of liquid dielectrics is due to the movement of ions that arise as a result of the dissociation of the liquid itself and impurities, as well as the movement of charged impurity particles - molions. With increasing temperature, the mobility of ions and the degree of thermal dissociation increase. These factors increase electrical conductivity.
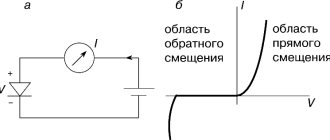
At low electric field strengths, Ohm's law comes into force, i.e. electric current I
, passing through the liquid, changes proportionally to the field strength.
In electric fields with high intensity E
(approximately 10...100 MV/m), the electric current does not obey Ohm's law due to the increase in the number of ions under the influence of the field.
In addition to ionic electrical conductivity, liquid dielectrics exhibit molion electrical conductivity, when charge carriers are tiny impurities. Such impurities can be water, various solid highly dispersed particles in suspension (fibers, dust particles, etc.). These particles adsorb ions on their surface and, when exposed to an electric field, move to the corresponding electrodes. In technical liquid dielectrics containing a certain proportion of impurities, molar conductivity predominates at room temperature. Molion conductivity is observed, for example, in transformer oil containing tiny particles of clay and emulsified water.
Purification of liquid dielectrics from impurities significantly increases their resistivity, but it is impossible to completely remove impurities.
The predominance of a particular type of conductivity depends on the dissociation energy, i.e., the energy required to destroy molecules and form ions. The higher the dissociation energy, the lower the ionic conductivity.
In polar liquids, dielectric losses consist of losses due to electrical conductivity and losses associated with dipole-relaxation polarization. They depend on temperature, frequency and viscosity of the liquid, since the rotation of dipoles in a viscous medium causes energy loss due to molecular friction. At high frequencies, liquid dielectrics have increased dielectric losses. For example, sovol at a temperature T-90°C and a frequency f
= 50 Hz has tanδ = 0.015. Therefore, liquid polar dielectrics are not recommended for use at high frequencies.
Dielectric losses in non-polar liquids that do not contain impurities are essentially losses due to electrical conductivity. They do not depend on frequency and increase with increasing temperature, which is explained by an increase in through current. The dielectric losses of these liquids are small, since their electrical conductivity is low. For example, pure transformer oil at temperature T = 90°C and frequency f = 50 Hz has tgδ = 0.003.
The electrical strength of liquid dielectrics is mainly determined by the presence of foreign impurities, liquid polarity, temperature and other factors. Gas bubbles present in the liquid are ionized, releasing energy that leads to local overheating of the liquid. This leads to the formation of a gas channel between the electrodes and, as a result, to breakdown of the liquid. In addition to gas inclusions, water is a significant factor that reduces the electrical strength of liquid dielectrics. Water droplets are polarized under the influence of an electric field and form chains with increased conductivity between the electrodes, along which electrical breakdown occurs. The electrical strength of liquids containing impurities is lower than that of purified ones.
Petroleum (mineral) oils (transformer, cable, capacitor), synthetic liquid dielectrics (chlorinated hydrocarbons, silicon and organofluorine liquids, esters of various types) and vegetable oils are used as liquid electrical insulating materials in electrical devices.
Petroleum electrical insulating
oils Petroleum oils are obtained through the process of stepwise distillation of oil and the removal of unstable compounds (naphthenic acids, sulfur, tar, oxygen, nitrogen, etc.) from the petroleum distillate (distillation product; for example, when distilling oil, distillates - gasoline, kerosene, lubricating oils, etc.) .
The technological operation of purifying petroleum distillate from foreign impurities is called refining. The performance properties of the oil depend to a certain extent on the quality of its implementation.
These oils have a number of properties that have made them widely used. They are relatively cheap and can be produced in large quantities, with good cleaning, they have a small dielectric loss tangent tgδ, and have a fairly high electrical strength.
The disadvantages of petroleum oils include a limited range of operating temperatures, fire and explosion hazards, and a tendency to aging.
When operating in a low-flow electrical apparatus, due to the oxidation of the corresponding fractions of hydrocarbons, the oil gradually ages, becoming darker. It produces partially soluble and insoluble polluting products. Insoluble heavy impurities settle on parts immersed in oil in the form of “sludge”, the viscosity of the oil increases, which impairs heat removal from heating parts. The aging process is accelerated by contact with air, especially if the air contains ozone; exposure to light and electric field; contact with certain metals (copper, iron, lead, etc.) and other substances with a crystalline structure; increased temperature, contact with rubber.
When aging in an electric field, some types of oils emit gases, which is dangerous, since gas bubbles can become sources of partial discharges. If the temperature of gaseous products (a mixture of oil vapor and air) exceeds their flash point, an explosion may occur.
The ability of oils not to emit gases when aging in an electric field (or even to absorb previously released gases) is called the gas resistance of oils.
To combat the aging of oils, the following means are used:
introduce antioxidant additives (substance inhibitors that slow down chemical reactions or stop them; used to slow down or prevent certain processes, such as metal corrosion, fuel oxidation (from the Latin inhibere - restrain, stop)), which easily combine with oxygen, protecting hydrocarbon fractions from oxidation, slow down the aging of oils and increase its service life; inhibitors are ionol, pyramidon, etc.;
limit the operating temperature (95°C for air-cooled transformers and 85°C for water-cooled transformers);
carry out continuous filtration of oils through adsorbents;
subject the aged oil to regeneration (Conversion of waste products into original ones for their reuse (from the Latin regeneratio - restoration, revival, renewal)), i.e. restoring its properties by cleaning and drying.
Transformer oil
- liquid from almost colorless to dark yellow. In terms of chemical composition, it is a mixture of naphthenic and paraffin hydrocarbons and therefore is a non-polar dielectric with low dielectric constant (ε = 2.2...2.3). After refining, the oil is further purified. To do this, it is treated with alkali, washed with water and dried. Final cleaning is carried out using adsorbents (Bodies on the surface of which a substance is absorbed from a solution or gas) (silica gel, infusorial earths) and filter presses.
Transformer oil has the following properties: low viscosity, which is very important, since too viscous oil removes heat loss from the windings and core of the transformer worse and impregnates porous insulation worse; pour point -70°C (which is especially important for equipment operating at low ambient temperatures);
electrical strength E
pr - 10...25 MV/m (very sensitive to moisture, but recovers upon drying);
The heat capacity and thermal conductivity of the oil increase with increasing temperature (with free convection (Transfer of heat, mass, charges by a moving medium, for example, air flows that arise naturally in a heterogeneous environment (natural convection) or created artificially (forced convection) (from the Latin convectio - import , carrying)) oil removes heat from the windings and transformer core immersed in it 25...30 times more intensely than air).
The main disadvantages of transformer oil are aging, flammability and combustibility, hygroscopicity (it is strictly forbidden to store it in open containers).
Transformer oil is used as an insulating and cooling medium in power and pulse transformers, reactors, high-voltage circuit breakers, such as arc extinguishing medium.
Cable oil
differs from transformer in increased viscosity, and from capacitor in reduced electrical properties. It is used as a component in oil-rosin compounds for impregnating the insulation of power cables.
Capacitor oil
obtained from transformer material after additional processing in a vacuum to remove dissolved air from it, which reduces dielectric losses. It is used to impregnate the insulation in paper and film capacitors, which makes it possible to reduce the overall dimensions, weight and cost of capacitors.
Synthetic liquid dielectrics. The use of synthetic liquid dielectrics is preferable in cases where their properties are superior to electrical insulating oils. For example, spruce requires the use of non-polar liquid dielectrics or liquid dielectrics with a higher fire and explosion hazard than electrical insulating oils.
Chlorinated hydrocarbons are produced by replacing some or even all of the hydrogen atoms with chlorine atoms in various hydrocarbons. The polar products of biphenyl chlorination are most often used. Chlorinated biphenyls, as well as the gases that are formed when these liquids are exposed to an electric arc, are toxic. Therefore, in a number of countries the use of chlorinated biphenyls for impregnation of capacitors is prohibited by law. The most famous representatives of this group are Sovol and Seutol-10. The atoms in the molecules of these materials are arranged asymmetrically, so Sovol and Seutol-10 are polar.
Sovol and sevtol-10 are slightly susceptible to aging, do not form explosive mixtures with air, are non-hygroscopic, toxic, and expensive.
Sovol (pentachlorobiphenyl) is a colorless viscous liquid obtained by chlorinating biphenyl (C12H10), as a result of which five hydrogen atoms of the latter are replaced by chlorine. Sovol is a non-flammable substance and does not oxidize, which is its main advantage over petroleum oils. However, its use is limited by the following disadvantages: density D
= 1500… 1560, flash point Tvsp = 205…230°C, pour point T3 = + 5°C, dielectric strength at temperature T= 20°C
E
pr = 14… 18 MV/m, significant viscosity and operating temperature ranges, which does not allow it to be used in its pure form; it is about 10 times more expensive than transformer oil.
It is used instead of capacitor oil for impregnation of low-voltage paper capacitors with increased capacity.
Sevtol-10 is a non-flammable liquid with a high pour point, which is obtained by diluting Sovol with trichlorobenzene. Used instead of transformer oil for explosive transformers.
Organosilicon liquids are a product of the synthesis of siliceous and carbon compounds, the properties of which are determined by the type of organic radicals. In accordance with this, polydimethylsiloxane, polydiethylsiloxane and polymethylphenylsiloxane liquids are distinguished.
These liquids are characterized by high heat resistance, low pour point, low temperature coefficient of viscosity, chemical inertness, low dielectric losses (dielectric loss tangent tgδ) and low hygroscopicity.
Polymethylsiloxane liquids are obtained by hydrolysis of dimethylchlorosilanes with trimethylchlorosilanes. They do not dissolve in alcohols and acetone, are highly inert and do not affect the properties of metals and rubbers upon contact with them. Used for impregnation of paper capacitors and hydrophobization of insulating tapes.
Polydiethylsiloxane liquids are mixtures of polyethylsiloxanes. Colorless. They are used for impregnation and filling of capacitors operating in the temperature range from -60 to 100°C.
Polymethylphenylsiloxane liquids are characterized by higher heat resistance and resistance to radiation.
The main properties of some electrical insulating liquids are given in Table 1.
Table 1. Basic properties of electrical insulating liquids
| Parameter | Sovol | Octol | Mineral oil | |
| transformer | capacitor | |||
| Density D, g/cm3 | 1,50… 1,56 | 0,85 | 0,84… 0,89 | 0,85. ..0,92 |
| Electrical resistivity p, Ohm-m | 1014… 10-15 | 1013.. 10-14 | 1014…1015 | 1014… 1016 |
| Dielectric constant ε at T = 20 °C | 5,0… 5,2 | 2,2… 2,4 | 2,1. ..2,4 | 2,1. ..2,3 |
| Dielectric loss tangent tgδ at | 0,002… | 0,005 | 0,0006… | 0,0003… 0,0006 |
| temperature 20 °C and frequency 50 Hz | …0,0004 | …0,001 | ||
| Electrical strength E at 20 °C | ||||
| frequency 50 Hz, kV/mm | 14.. .18 | 12.. .15 | 15.. .20 | 20… 25 |
| Temperature, °C: | ||||
| | hardening, no more | -8 | -15 | -45 | -45 |
| flashes, no less | 200… 230 | |||
| Acid number, mg KOH/g oil | 0,01. ..0,02 | 0,03 | 0,03… 0,05 | 0,015. ..1,020 |
Organofluorine liquids
are derivatives of hydrocarbons in which the hydrogen atoms are replaced by fluorine. Their vapors do not form explosive mixtures with air. They have low dielectric losses (dielectric loss tangent tgδ), negligible hygroscopicity, high heat resistance (some liquids can operate for a long time at temperatures of 200 °C and above), high thermal conductivity, complete non-flammability, and high arc resistance.
Organofluorine liquids are used for impregnation and filling of capacitors and transformers, for testing radio electronics elements at low and high temperatures.
In addition to the indicated liquid dielectrics, highly polar synthetic electrical insulating liquids are used in radio electronics. For example, ethylene glycol (HO - CH2 - CH2 - OH), which is used as a control liquid when monitoring the tightness of microcircuits.
Equipment and accessories: refractometer, set of flasks with solutions, glass rod, filter paper.
Liquid dielectrics are low-molecular substances of organic origin, which can be polar or non-polar. Their electrical properties largely depend on the structure of the molecules and the presence of impurities. Impurities are formed during the oxidation and decomposition of hydrocarbon fractions, during the absorption of water and the ingress of particles of fibrous materials.
Liquid dielectrics are characterized by dielectric constant ε, electrical conductivity, dielectric losses (dielectric loss tangent tgδ), electrical strength E.
For polar liquids (Sovol, hexol, ethylene glycol), the dielectric constant ε is determined simultaneously by electronic and dipole polarizations. For example, for hexol ε = 3, for ethylene glycol ε = 40.
In non-polar liquids, the dielectric constant is determined mainly only by electronic polarization, does not depend on frequency and decreases with increasing temperature, approaching unity.
This phenomenon is explained by a decrease in the number of molecules per unit volume. Non-polar liquids have ε less than polar liquids. For example, for carbon tetrachloride ε = 2.163, for toluene ε = 2.294.
The electrical conductivity of liquid dielectrics is due to the movement of ions that arise as a result of the dissociation of the liquid itself and impurities, as well as the movement of charged impurity particles - molions. With increasing temperature, the mobility of ions and the degree of thermal dissociation increase. These factors increase electrical conductivity.
At low electric field strengths, Ohm's law comes into force, i.e. electric current I
, passing through the liquid, changes proportionally to the field strength.
In electric fields with high intensity E
(approximately 10...100 MV/m), the electric current does not obey Ohm's law due to the increase in the number of ions under the influence of the field.
In addition to ionic electrical conductivity, liquid dielectrics exhibit molion electrical conductivity, when charge carriers are tiny impurities. Such impurities can be water, various solid highly dispersed particles in suspension (fibers, dust particles, etc.). These particles adsorb ions on their surface and, when exposed to an electric field, move to the corresponding electrodes. In technical liquid dielectrics containing a certain proportion of impurities, molar conductivity predominates at room temperature. Molion conductivity is observed, for example, in transformer oil containing tiny particles of clay and emulsified water.
Purification of liquid dielectrics from impurities significantly increases their resistivity, but it is impossible to completely remove impurities.
The predominance of a particular type of conductivity depends on the dissociation energy, i.e., the energy required to destroy molecules and form ions. The higher the dissociation energy, the lower the ionic conductivity.
In polar liquids, dielectric losses consist of losses due to electrical conductivity and losses associated with dipole-relaxation polarization. They depend on temperature, frequency and viscosity of the liquid, since the rotation of dipoles in a viscous medium causes energy loss due to molecular friction. At high frequencies, liquid dielectrics have increased dielectric losses. For example, sovol at a temperature T-90°C and a frequency f
= 50 Hz has tanδ = 0.015. Therefore, liquid polar dielectrics are not recommended for use at high frequencies.
Dielectric losses in non-polar liquids that do not contain impurities are essentially losses due to electrical conductivity. They do not depend on frequency and increase with increasing temperature, which is explained by an increase in through current. The dielectric losses of these liquids are small, since their electrical conductivity is low. For example, pure transformer oil at temperature T = 90°C and frequency f = 50 Hz has tgδ = 0.003.
The electrical strength of liquid dielectrics is mainly determined by the presence of foreign impurities, liquid polarity, temperature and other factors. Gas bubbles present in the liquid are ionized, releasing energy that leads to local overheating of the liquid. This leads to the formation of a gas channel between the electrodes and, as a result, to breakdown of the liquid. In addition to gas inclusions, water is a significant factor that reduces the electrical strength of liquid dielectrics. Water droplets are polarized under the influence of an electric field and form chains with increased conductivity between the electrodes, along which electrical breakdown occurs. The electrical strength of liquids containing impurities is lower than that of purified ones.
Petroleum (mineral) oils (transformer, cable, capacitor), synthetic liquid dielectrics (chlorinated hydrocarbons, silicon and organofluorine liquids, esters of various types) and vegetable oils are used as liquid electrical insulating materials in electrical devices.
Petroleum electrical insulating
oils Petroleum oils are obtained through the process of stepwise distillation of oil and the removal of unstable compounds (naphthenic acids, sulfur, tar, oxygen, nitrogen, etc.) from the petroleum distillate (distillation product; for example, when distilling oil, distillates - gasoline, kerosene, lubricating oils, etc.) .
The technological operation of purifying petroleum distillate from foreign impurities is called refining. The performance properties of the oil depend to a certain extent on the quality of its implementation.
These oils have a number of properties that have made them widely used. They are relatively cheap and can be produced in large quantities, with good cleaning, they have a small dielectric loss tangent tgδ, and have a fairly high electrical strength.
The disadvantages of petroleum oils include a limited range of operating temperatures, fire and explosion hazards, and a tendency to aging.
When operating in a low-flow electrical apparatus, due to the oxidation of the corresponding fractions of hydrocarbons, the oil gradually ages, becoming darker. It produces partially soluble and insoluble polluting products. Insoluble heavy impurities settle on parts immersed in oil in the form of “sludge”, the viscosity of the oil increases, which impairs heat removal from heating parts. The aging process is accelerated by contact with air, especially if the air contains ozone; exposure to light and electric field; contact with certain metals (copper, iron, lead, etc.) and other substances with a crystalline structure; increased temperature, contact with rubber.
When aging in an electric field, some types of oils emit gases, which is dangerous, since gas bubbles can become sources of partial discharges. If the temperature of gaseous products (a mixture of oil vapor and air) exceeds their flash point, an explosion may occur.
The ability of oils not to emit gases when aging in an electric field (or even to absorb previously released gases) is called the gas resistance of oils.
To combat the aging of oils, the following means are used:
introduce antioxidant additives (substance inhibitors that slow down chemical reactions or stop them; used to slow down or prevent certain processes, such as metal corrosion, fuel oxidation (from the Latin inhibere - restrain, stop)), which easily combine with oxygen, protecting hydrocarbon fractions from oxidation, slow down the aging of oils and increase its service life; inhibitors are ionol, pyramidon, etc.;
limit the operating temperature (95°C for air-cooled transformers and 85°C for water-cooled transformers);
carry out continuous filtration of oils through adsorbents;
subject the aged oil to regeneration (Conversion of waste products into original ones for their reuse (from the Latin regeneratio - restoration, revival, renewal)), i.e. restoring its properties by cleaning and drying.
Transformer oil
- liquid from almost colorless to dark yellow. In terms of chemical composition, it is a mixture of naphthenic and paraffin hydrocarbons and therefore is a non-polar dielectric with low dielectric constant (ε = 2.2...2.3). After refining, the oil is further purified. To do this, it is treated with alkali, washed with water and dried. Final cleaning is carried out using adsorbents (Bodies on the surface of which a substance is absorbed from a solution or gas) (silica gel, infusorial earths) and filter presses.
Transformer oil has the following properties: low viscosity, which is very important, since too viscous oil removes heat loss from the windings and core of the transformer worse and impregnates porous insulation worse; pour point -70°C (which is especially important for equipment operating at low ambient temperatures);
electrical strength E
pr - 10...25 MV/m (very sensitive to moisture, but recovers upon drying);
The heat capacity and thermal conductivity of the oil increase with increasing temperature (with free convection (Transfer of heat, mass, charges by a moving medium, for example, air flows that arise naturally in a heterogeneous environment (natural convection) or created artificially (forced convection) (from the Latin convectio - import , carrying)) oil removes heat from the windings and transformer core immersed in it 25...30 times more intensely than air).
The main disadvantages of transformer oil are aging, flammability and combustibility, hygroscopicity (it is strictly forbidden to store it in open containers).
Transformer oil is used as an insulating and cooling medium in power and pulse transformers, reactors, high-voltage circuit breakers, such as arc extinguishing medium.
Cable oil
differs from transformer in increased viscosity, and from capacitor in reduced electrical properties. It is used as a component in oil-rosin compounds for impregnating the insulation of power cables.
Capacitor oil
obtained from transformer material after additional processing in a vacuum to remove dissolved air from it, which reduces dielectric losses. It is used to impregnate the insulation in paper and film capacitors, which makes it possible to reduce the overall dimensions, weight and cost of capacitors.
Synthetic liquid dielectrics. The use of synthetic liquid dielectrics is preferable in cases where their properties are superior to electrical insulating oils. For example, spruce requires the use of non-polar liquid dielectrics or liquid dielectrics with a higher fire and explosion hazard than electrical insulating oils.
Chlorinated hydrocarbons are produced by replacing some or even all of the hydrogen atoms with chlorine atoms in various hydrocarbons. The polar products of biphenyl chlorination are most often used. Chlorinated biphenyls, as well as the gases that are formed when these liquids are exposed to an electric arc, are toxic. Therefore, in a number of countries the use of chlorinated biphenyls for impregnation of capacitors is prohibited by law. The most famous representatives of this group are Sovol and Seutol-10. The atoms in the molecules of these materials are arranged asymmetrically, so Sovol and Seutol-10 are polar.
Sovol and sevtol-10 are slightly susceptible to aging, do not form explosive mixtures with air, are non-hygroscopic, toxic, and expensive.
Sovol (pentachlorobiphenyl) is a colorless viscous liquid obtained by chlorinating biphenyl (C12H10), as a result of which five hydrogen atoms of the latter are replaced by chlorine. Sovol is a non-flammable substance and does not oxidize, which is its main advantage over petroleum oils. However, its use is limited by the following disadvantages: density D
= 1500… 1560, flash point Tvsp = 205…230°C, pour point T3 = + 5°C, dielectric strength at temperature T= 20°C
E
pr = 14… 18 MV/m, significant viscosity and operating temperature ranges, which does not allow it to be used in its pure form; it is about 10 times more expensive than transformer oil.
It is used instead of capacitor oil for impregnation of low-voltage paper capacitors with increased capacity.
Sevtol-10 is a non-flammable liquid with a high pour point, which is obtained by diluting Sovol with trichlorobenzene. Used instead of transformer oil for explosive transformers.
Organosilicon liquids are a product of the synthesis of siliceous and carbon compounds, the properties of which are determined by the type of organic radicals. In accordance with this, polydimethylsiloxane, polydiethylsiloxane and polymethylphenylsiloxane liquids are distinguished.
These liquids are characterized by high heat resistance, low pour point, low temperature coefficient of viscosity, chemical inertness, low dielectric losses (dielectric loss tangent tgδ) and low hygroscopicity.
Polymethylsiloxane liquids are obtained by hydrolysis of dimethylchlorosilanes with trimethylchlorosilanes. They do not dissolve in alcohols and acetone, are highly inert and do not affect the properties of metals and rubbers upon contact with them. Used for impregnation of paper capacitors and hydrophobization of insulating tapes.
Polydiethylsiloxane liquids are mixtures of polyethylsiloxanes. Colorless. They are used for impregnation and filling of capacitors operating in the temperature range from -60 to 100°C.
Polymethylphenylsiloxane liquids are characterized by higher heat resistance and resistance to radiation.
The main properties of some electrical insulating liquids are given in Table 1.
Table 1. Basic properties of electrical insulating liquids
| Parameter | Sovol | Octol | Mineral oil | |
| transformer | capacitor | |||
| Density D, g/cm3 | 1,50… 1,56 | 0,85 | 0,84… 0,89 | 0,85. ..0,92 |
| Electrical resistivity p, Ohm-m | 1014… 10-15 | 1013.. 10-14 | 1014…1015 | 1014… 1016 |
| Dielectric constant ε at T = 20 °C | 5,0… 5,2 | 2,2… 2,4 | 2,1. ..2,4 | 2,1. ..2,3 |
| Dielectric loss tangent tgδ at | 0,002… | 0,005 | 0,0006… | 0,0003… 0,0006 |
| temperature 20 °C and frequency 50 Hz | …0,0004 | …0,001 | ||
| Electrical strength E at 20 °C | ||||
| frequency 50 Hz, kV/mm | 14.. .18 | 12.. .15 | 15.. .20 | 20… 25 |
| Temperature, °C: | ||||
| | hardening, no more | -8 | -15 | -45 | -45 |
| flashes, no less | 200… 230 | |||
| Acid number, mg KOH/g oil | 0,01. ..0,02 | 0,03 | 0,03… 0,05 | 0,015. ..1,020 |
Organofluorine liquids
are derivatives of hydrocarbons in which the hydrogen atoms are replaced by fluorine. Their vapors do not form explosive mixtures with air. They have low dielectric losses (dielectric loss tangent tgδ), negligible hygroscopicity, high heat resistance (some liquids can operate for a long time at temperatures of 200 °C and above), high thermal conductivity, complete non-flammability, and high arc resistance.
Organofluorine liquids are used for impregnation and filling of capacitors and transformers, for testing radio electronics elements at low and high temperatures.
In addition to the indicated liquid dielectrics, highly polar synthetic electrical insulating liquids are used in radio electronics. For example, ethylene glycol (HO - CH2 - CH2 - OH), which is used as a control liquid when monitoring the tightness of microcircuits.
Equipment and accessories: refractometer, set of flasks with solutions, glass rod, filter paper.
Lecture 10 Gaseous, liquid and solid dielectrics. - presentation
Lecture 10 Gaseous, liquid and solid dielectrics.
Lecture questions: Gaseous dielectrics, main characteristics, electronegative gases, application in energy. Liquid dielectrics. Application in energy general properties of used and promising liquid dielectrics General characteristics of dielectrics Types of dielectrics. Application of solid dielectrics Properties of the most commonly used dielectrics Polymer materials Paper and cardboard Materials for insulators Mica materials.
Gaseous dielectrics. Main characteristics. The main characteristics of gases as dielectrics are dielectric constant, electrical conductivity, and electrical strength. In addition, thermophysical characteristics, primarily thermal conductivity, are often important. Dielectric constant of gases: = 1+n( +3 2 /kT)/ 0 where n is the number of molecules with polarizability and dipole moment per unit volume. Usually the value is close to 1, the difference from unity can be found in 3-4 decimal places. The reason for this is the small number n of molecules in the gas phase.
The electrical conductivity of gases is usually no worse than S/m, and the main factor causing conductivity in not very strong fields is ionizing radiation. Dielectric losses are insignificant and should be taken into account only in the region of strong fields. The electrical strength of gases, compared with the strength of liquids and solid dielectrics, is low and strongly depends on both external conditions and the nature of the gas. Typically, the breakdown characteristics of different gases are compared under normal conditions (n.s.),
pressure 1 atm., temperature 20 C, electrodes creating a uniform field, area 1 cm 2, interelectrode gap 1 cm. Air at no. has an electrical strength of 30 kV/cm. The coefficient k, showing the ratio of the electrical strength of gas to the electrical strength of air, is for some gases: hydrogen k = 0.5, helium k = 0.2, SF6 gas k = 2.9, freon-12 k = 2.4, perfluorinated hydrocarbon gases k = (4-10).
The thermal conductivity of gases is also small compared to the thermal conductivity of solids and liquids; its highest value = 0.2 W/(m K) is for hydrogen. For the most popular gases = 0.03 W/(m K) - air, = W/(m K) - SF6 gas. For comparison, aluminum = 200 W/(m K). The maximum operating temperatures of gases are determined either by the decomposition of gas molecules or by an increase in electrical conductivity due to the dissociation of gas molecules under the influence of thermal energy. Typical temperatures for the second option are on the order of more than a thousand degrees.
Electronegative gases, use of gaseous dielectrics. Air is the most widely used gas in the energy sector. This is due to the low cost and general availability of air, the ease of creation, maintenance and repair of air electrical insulation systems, and the possibility of visual inspection. Objects that use air as electrical insulation - power lines, open switchgear, air circuit breakers, etc.
Electronegative gases are gases whose molecules have an affinity for electrons, which means that when they capture an electron and convert the molecule into a negative ion, energy is released. This process leads to the phenomenon of electron sticking, and thereby reducing the effective impact ionization coefficient by the value of the sticking coefficient eff. Therefore, electronegative gases have increased electrical strength. Of the electronegative gases with high electrical strength, SF6 gas has found the most application. It gets its name from the abbreviation electric gas. The unique properties of SF6 gas were discovered in Russia, and its use also began in Russia.
The electrical strength of SF6 gas at atmospheric pressure and a gap of 1 cm is E = 89 kV/cm. Molecular weight 146, characterized by a very large coefficient of thermal expansion and high density. This is important for power plants in which any parts of the device are cooled, because with a large coefficient of thermal expansion, a convective flow easily forms, carrying away heat. From thermophysical properties: melting point = -50 C at 2 atm, boiling point (sublimation) = -63 C. Low values of the latter parameters mean the possibility of using SF6 gas at low temperatures.
Other useful properties of SF6 gas: - chemical inertness, - non-toxicity, - non-flammability, - heat resistance (up to 800 C), - explosion safety, - weak decomposition in discharges, - low liquefaction temperature. In the absence of impurities, SF6 gas is completely harmless to humans. However, the decomposition products of SF6 gas as a result of discharges (for example, in a spark gap or switch) are toxic and chemically active.
Application: In devices, SF6 gas is usually used under pressure of several atmospheres. cables, capacitors, switches, compact switchgear (closed switchgear). SF6 gas is most widely used abroad, especially in Japan. For example, the use of SF6 gas makes it possible to reduce the size of switchgears by tens of times, which is very important given the high cost of land for locating energy facilities. This is profitable even despite the high cost of SF6 gas - more than $10 per 1 kilogram.
Liquid dielectrics. General properties. From an electrophysical point of view, the most important characteristics of liquids are dielectric constant, electrical conductivity and dielectric strength. Dielectric constant is a true characteristic of liquids and is characterized by the dipole moment and polarizability of molecules. For example, the nonpolar dielectric hexane has no dipole moment, polarization is purely electronic in nature and, as a result, the dielectric constant is low.
Transformer oil, being a mixture of substances, contains a small amount of polar molecules that have a dipole moment. Therefore it increases to ~.2-2.4. Castor oil has more polar molecules, hence more than ~4.5. Ethyl alcohol, glycerin, water are representatives of polar substances, ~ 24, 40, 81, respectively. For non-polar liquids
The electrical conductivity of liquids is determined by the ionization of molecules, the presence of special types of impurities in the liquid: ionophores and ionogens, and the occurrence of electrohydrodynamic flows. Purification of dielectric liquids can be carried out by distillation, incl. under vacuum, partial crystallization, adsorption, ion exchange. In this case, as a rule, electrical conductivity and dielectric losses decrease, and electrical strength increases.
The main impurity that gives conductivity to liquid dielectrics is water, and the main impurities that reduce the electrical strength are microparticles, microbubbles and water. The electrical conductivity of liquids increases most radically (up to 6 orders of magnitude compared to data from reference books) after using a new purification method - electrodialysis. Electrodialysis is a method of removing ions from a gap by passing a direct current using ion exchange membranes, the conductivity of which is carried out by only one type of ion.
Electrical strength, like electrical conductivity, is to a large extent a technological characteristic of the liquid dielectric and electrodes, methods of preparation and operation of the insulating gap. There are several of the most common and obvious methods for increasing the electrical strength: - degassing the liquid, - passing through an adsorbent, - passing through a filter with submicron pore sizes. Some of these methods are used in power systems for oil drying and recovery.
Whether electrical strength is a true characteristic of a liquid is a rather fundamental question. In our opinion, electrical breakdown is a consequence of a chain of events that are very sensitive to both impurities and the properties of the electrode-liquid interface. Therefore, the breakdown can be controlled. Example: breakdown at a constant voltage of a dielectric liquid - perfluorotriethylamine (C 2 F 5 ) 3 N. The first measurements of fresh liquid without cleaning the liquid and electrodes gave Epr = kV/cm. If the liquid is subjected to degassing, dehydration and filtration operations, kV/cm can be obtained. After an additional training series in low-power discharges, the electrical strength reached kV/cm. That is, an increase of almost 10 times!
Current and promising liquid dielectrics. The most common liquid dielectric in the energy sector is transformer oil (TO). Transformer oil is a purified fraction of oil obtained during distillation, boiling at a temperature of 300 C to 400 C. Depending on the origin of the oil, T.M. has various properties. It has a complex hydrocarbon composition with an average molecular weight of a.e.
Transformer oil contains the following main components. 1. Paraffins 10-15% 2. Naphthenes or cycloparaffins 60-70% 3. Aromatic hydrocarbons 15-20% 4. Asphalt-resin substances 1-2% 5. Sulfur compounds
Paraffins and cycloparaffins provide low electrical conductivity and high electrical strength. Aromatic hydrocarbons reduce oil aging and increase resistance to partial discharges in the oil volume. Asphalt-resin, sulfur, nitrogen compounds and naphthenic acids are impurities and do not play a positive role. Asphalt-resin compounds are responsible for the formation of sediment in the oil and its color. Sulfur and nitrogen compounds and naphthenic acids are responsible for the corrosion processes of metals in transformer oil.
Paraffin hydrocarbons, in addition to high chemical stability, have a high flash point and a number of other positive qualities, but they lose fluidity (solidify) already at room temperature and therefore a high paraffin content is not allowed. Moreover, oils with a high content (Grozny, Surakhani) are not used for preparing oils. Naphthenic hydrocarbons are less stable than paraffins and are easily oxidized. A typical naphthenic oil is Dossor oil, from which the best transformer oil is prepared.
Aromatic hydrocarbons are divided into hydrocarbons with a symmetrical structure (benzene, naphthalene, anthracene) and aromatics with long side chains (toluene). The former are one of the most difficult substances to oxidize. These aromatics are a valuable component of the oil, as they protect it from oxidation. The latter combine very easily with oxygen, and their ability to self-oxidize increases with the number and length of side chains.
Basic physical and chemical properties of oil. Combustible, biodegradable, virtually non-toxic, and does not deplete the ozone layer. The density of the oil is usually in the range ( ) 10 3 kg/m 3. Viscosity is one of the most important properties of the oil. From the standpoint of high electrical strength, it is desirable to have an oil of higher viscosity. In order to perform its additional functions well in transformers (as a cooling medium) and switches (as a medium where drive elements move), the oil must have a low viscosity, otherwise the transformers will not cool properly and the switches will not break the electric arc at the time set for them.
The pour point is the temperature at which the oil thickens so much that when a test tube with cooled oil is tilted at an angle of 45, its level will remain unchanged for 1 minute. In oil switches, the pour point is critical. Fresh oil should not harden at a temperature of -45 C; in the southern regions of the country it is allowed to use oil with a pour point of -35 C. The flash point is the temperature of the oil heated in a crucible, at which its vapor forms a mixture with air that ignites when a flame is applied to it. The flash occurs so quickly that the oil does not have time to warm up and ignite. The flash point of transformer oil should not be lower than 135 C. If the oil is heated above the flash point, then the temperature at which the oil ignites and burns for at least 5 seconds is called the ignition temperature of the oil. The temperature at which combustion occurs in a closed crucible, in the presence of air, without the application of a flame, is called the auto-ignition temperature. For transformer oil it is C
Among other thermophysical characteristics, we note the relatively low thermal conductivity from 0.09 to 0.14 W/(m K), Heat capacity - from 1.5 kJ/(kG K) to 2.5 k J/(kG K). The coefficient of thermal expansion of the oil determines the size requirements for the transformer expansion tank and is approximately /K.
Oil resistivity is standardized at a temperature of 90 C and a field strength of 0.5 MV/m, and it should not exceed Ohm m for any grade of oil. The resistivity, as well as the viscosity, drops significantly with increasing temperature (by more than an order of magnitude when the temperature decreases by 50 C). The dielectric constant of the oil is small and fluctuates within the limits. The tangent of the dielectric loss angle is determined by the presence of impurities in the oil. In pure oil it should not exceed at a temperature of 90 C and an operating frequency of 50 Hz. In oxidized, contaminated and moistened oil, tg increases and can reach more than 0.2. The electrical strength of the oil is determined in a standard spark gap with hemispherical electrodes with a diameter of 25.4 mm and an interelectrode distance of 2.5 mm. The breakdown voltage must be at least 70 kV, while in the spark gap the electrical strength of the oil will be at least 280 kV/cm.
A transformer can operate without repair for years, but the oil requires cleaning after just a year, and regeneration after 4-5 years. Measures to extend the life of the oil are: 1) protecting the oil from contact with outside air by installing expanders with filters that absorb oxygen and water, as well as displacing air from the oil; 2) reduction of oil overheating under operating conditions; 3) regular cleaning of water and sludge; 4) use of continuous oil filtration to reduce acidity; 5) introduction of antioxidants.
Condenser oils. This term combines a group of various dielectrics used for impregnation of paper-oil and paper-film insulation of capacitors. The most common capacitor oil according to GOST is produced from transformer oil through deeper purification. It differs from conventional oils in greater transparency and a lower tg value (more than ten times).
Castor oil is of vegetable origin, it is obtained from castor bean seeds. The main area of use is the impregnation of paper capacitors for operation under pulsed conditions. dielectric constant at 20 C is 4.0 - 4.5; tg at 20 C is equal to 0.01 - 0.03, Epr at 20 C is equal to MV/m. This dielectric belongs to weakly polar liquid dielectrics; its resistivity under normal conditions is 10 8 – Ohm m.
Cable oils are intended for impregnation of paper insulation of power cables. They are also based on petroleum oils. They differ from transformer oil by increased viscosity, increased flash point and reduced dielectric losses.).
The second type of liquid dielectrics are low-flammable and non-flammable liquids. Chlorobiphenyls (chlorinated biphenyls) are most widely used in energy and electrical engineering. Chlorobiphenyls are nonflammable and good dielectrics (= 5-6) due to the polarity of the bond between the electronegative chlorine and the diphenyl ring. The dielectric loss tangent tg is not much higher than that of oil, and the dielectric strength is also high. Application: transformers and other electrical devices filled with chlorodiphenyl dielectrics in fire hazardous conditions. Disadvantages: high toxicity and strong impact on the ozone layer.
In this regard, they are trying to find a replacement for chlorobiphenyls. For example, in Russia and some other countries, silicones (siloxanes) or organosilicon liquids are considered the most promising for use. This is a class of liquids with different electrical and thermophysical characteristics. Well-purified liquids have = , tg Ohm m. Typically, these compounds have an increased flash point (up to 300C). Disadvantages: The studied organosilicon liquids cannot provide fire safety and, therefore, cannot completely replace chlorinated biphenyls. In addition, they are several times more expensive than transformer oil.
Organofluorine liquids (perfluorocarbons). Fluorocarbon liquids are inert to any influences, incl. stable under the influence of electric field and temperature. They do not dissolve oils, rubber, water, etc. Properties: - non-flammable; — high thermal and chemical stability; - non-toxic, colorless and odorless; — the ability to select liquids with different boiling and freezing points; — low solubility of water and high solubility of gases; — lack of solubility of any non-fluorinated materials; — high coefficient of thermal expansion.
According to electrophysical parameters: = 1.8-2, tg ( ) Ohm m, electrical strength - up to 500 kV/cm, high electrical strength in the gaseous (vapor) state - up to kV/cm, non-flammability, thermal stability up to a temperature of more than 400, organofluorine liquids significantly exceed similar performance of any other liquids, including mineral oils. They are non-toxic, non-oxidizing, have low viscosity, incl. in the low temperature region. A number of liquids have a freezing point of -70 C or lower. The main obstacle to wider use is the relatively high price.
General characteristics of solid dielectrics A nonpolar dielectric is a substance containing molecules with predominantly covalent bonds. A polar dielectric is a substance containing dipole molecules or groups, or having ions in its structure. A ferroelectric is a substance containing regions with spontaneous polarization.
Their polarization mechanisms differ sharply: - purely electronic polarization for non-polar dielectrics such as polyethylene, polystyrene, while it is small, no more than 3, dielectric losses are also small; - ionic polarization in ionic crystals such as NaCl or dipole in polar dielectrics such as ice, which can range from 3-4 to 100, dielectric losses can be very significant, especially at dipole rotation frequencies and other resonant frequencies;
- domain polarization in ferroelectrics is maximum and can reach , dielectric losses can be very significant, especially at resonant frequencies and in the region of higher frequencies. Features of conductivity mechanisms in solid dielectrics - the concentration of carriers is very low, the mobility of ions in homogeneous materials is very small, the mobility of electrons in pure materials is high, in technically pure materials it is small.
Some terms specific to solid dielectrics: Chemical resistance - the ability to withstand contacts with different environments (acid - acid resistance, alkali - alkali resistance, ozone - ozone resistance, oil - oil resistance, water - water resistance); Tracking resistance - the ability to withstand the action of an arc; Dendrite resistance - the ability to resist the formation of dendrites
Types of dielectrics. Application of solid dielectrics in energy: All dielectric materials can be divided into groups using different principles. For example, divide into inorganic and organic materials. Inorganic dielectrics. glass, mica, ceramics, inorganic films (oxides, nitrides, fluorides), metal phosphates, electrical insulating concrete. The features of inorganic dielectrics are that they are non-flammable, usually light-, ozone-, heat-resistant, and have complex manufacturing technology. There is practically no aging at alternating voltage; they are prone to aging at constant voltage.
Organic dielectrics: polymers, waxes, varnishes, rubbers, papers, varnished fabrics. Features of organic dielectrics are that they are (mostly) flammable, low-resistant to atmospheric and operational influences, have (mostly) simple manufacturing technology, and are usually cheaper compared to inorganic dielectrics. Aging at constant voltage is practically absent; at alternating voltage they age due to partial discharges, dendrites and water trees.
Application in the energy sector: - linear and substation insulation - porcelain, glass and silicone rubber in overhead insulators of overhead lines, porcelain in support and bushing insulators, fiberglass as load-bearing elements, polyethylene, paper in high-voltage bushings, paper, polymers in power cables; - insulation of electrical devices - paper, getinax, fiberglass, polymers, mica materials;
- machines, devices - paper, cardboard, varnishes, compounds, polymers; - different types of capacitors - polymer films, paper, oxides, nitrides. From a practical point of view, in each case of choosing an electrical insulation material, the operating conditions should be analyzed and the insulation material should be selected in accordance with a set of requirements.
For orientation, it is advisable to divide the main dielectric materials into groups according to application conditions. Heat-resistant electrical insulation (products made of mica materials, glass and materials based on them, organosilicate, metal phosphate coatings, ceramic materials, organosilicon compositions with a heat-resistant binder). Moisture-resistant electrical insulation - these materials must be hydrophobic (not wetted by water) and non-hygroscopic.
Radiation-resistant insulation: inorganic films, ceramics, fiberglass, mica materials, some types of polymers (polyimides, polyethylene). Tropical-resistant insulation. The material must be hydrophobic to work in conditions of high humidity and temperature. In addition, it must be resistant to mold fungi. The best materials: fluoroplastic, some other polymers, the worst - paper, cardboard.
Frost-resistant insulation. This requirement is typical mainly for rubbers, because When the temperature drops, all rubbers lose their elasticity. Organosilicon rubber with phenyl groups is the most frost-resistant (up to -90 C). Insulation for work in vacuum (space, vacuum devices). For these conditions it is necessary to use vacuum-tight materials. Some specially prepared ceramic materials are suitable; polymers are of little use.
Properties of the most commonly used dielectrics. Polymer materials Polymers are good dielectrics. They have: low dielectric losses, high resistivity, high electrical strength, high manufacturability and, as a rule, low price. In addition, based on polymers with dispersed additives, it is possible to obtain a variety of composite materials with a wide range of properties.
Based on technological characteristics, polymer materials are divided into 2 classes - thermoplastics and thermosets. Thermoplastics - soften when heated, while granules of the original polymer are placed in the chamber of a thermoplastic machine, heated to the softening temperature, pressed and cooled. This is how small dielectric parts are made. For large-sized products, such as cables, together with the internal cable electrode. The most common dielectric of this class is polyethylene H-(CH 2 ) n H.
Its main parameters: resistivity Ohm m, specific surface resistance Ohm, dielectric constant, dielectric loss tangent 10 -4, electrical strength kV/mm, thermal conductivity W/(m K), heat capacity 2 kJ/(kg K), density kg/m 3. Heat resistance class Y. Polyethylene is widely used as power electrical insulation in cables, especially the so-called. "cross-linked" polyethylene. (In foreign literature - cross-linked polyethylene).
Thermosets do not soften when heated; after reaching a certain temperature, they begin to collapse. Products from them are usually made by pressing heated powders, or by polymerization directly in the product. Thermosets based on phenol-formaldehyde polymers (bakelite) and amino-formaldehyde polymers are quite cheap and technologically advanced. Their electrical characteristics are low.
Epoxy polymers have good mechanical strength and satisfactory electrical characteristics. They are polar dielectrics. High polarity results in poor water resistance. The main advantage of epoxy compounds is the simplicity of the preparation technology. Among other thermoset polymers, we note a dielectric material with high mechanical strength - caprolon, with a wide range of operating temperatures (-100 C to +250 C) - polyimides and composites based on them.
Paper and cardboard The thinnest and strongest papers are used to make capacitors. It is enough to note that the density of condenser papers reaches 1.6 t/m3, i.e. more than 1.5 times the density of water. In this case, the electrical strength of paper 10 microns thick, impregnated with transformer oil, is up to 10 MV/cm.
Electrical cardboard is used as dielectric spacers, washers, spacers, etc. Cardboard is usually used after impregnation with transformer oil. The electrical strength of impregnated cardboard reaches kV/mm. Oil barrier insulation usually has a strength of E = kV/cm. The disadvantage of cardboard is its hygroscopicity
Materials for insulators. Recently, the production of insulators for overhead lines based on silicone rubber has been rapidly developing. This material belongs to rubbers, the main property of which is elasticity. Different types of rubbers are used in the energy sector: natural rubbers, butadiene rubbers, styrene butadiene rubbers, ethylene propylene rubbers and organosilicon rubbers.
Organosilicon rubbers are based on polyorganosiloxanes: RR | | HO-Si-O-{-Si-O-} n H | | RR Where R are the same or different organic radicals. Depending on the type of these radicals, the properties of organosilicon rubber change. The properties of pure organosilicon rubbers are unsatisfactory, primarily due to low strength and insufficient light-ozone resistance. Nanopowders of silicon dioxide (aerosil, white soot) and titanium dioxide are used as reinforcing active fillers.
From the electrical and thermophysical properties of the composite material, we note: dielectric constant = ; specific volume resistivity Ohm m; specific surface resistance Ohm; dielectric loss tangent;
electrical strength kV/mm, heat capacity kJ/(kg K); density kg/m 3 ; tensile strength 4-6 MPa. Properties of silicone rubber: high heat capacity, relatively low mechanical strength, resistance to ozone, light and oil, frost resistance ( ) C and heat resistance ( ) C, moisture resistance, but gas permeability, oil-gasoline resistance.
Electrical porcelain Among its most valuable properties is its high resistance to atmospheric influences, positive and negative temperatures, to the effects of chemical reagents, high mechanical and electrical strength, and low cost of initial components. This determined the widespread use of porcelain for the production of insulators. The disadvantages of porcelain are fragility, high density, low thermal conductivity, and high dielectric losses.
The main parameters of porcelain: dielectric constant at 50 Hz 5.0-7.0 E pr at 50 Hz MV/m specific volume resistivity at 20 0 C Ohm m; 10 3 tg at 50 Hz: 25-30; specific heat capacity at C J/(kg k)
Electrical glass as a material for insulators has some advantages over porcelain. In particular, it has a more stable raw material base, simpler technology that allows for greater automation, and the ability to visually monitor faulty insulators. In terms of chemical composition, glass is a set of oxides of silicon, boron, aluminum, sodium, calcium, etc. According to its thermodynamic state, it is a highly thickened liquid due to supercooling.
Main parameters of glass: dielectric constant 4.8-8.2 specific volume resistance Ohm m; tg at Hz: (5-250)10 -4; specific heat capacity 0.3-1.0 kJ/(kg s)
Ordinary alkali glass is unsuitable for the manufacture of insulators due to cracking, clouding, etc. under operating conditions. For this purpose, special low-alkaline (sodium and potassium-free) glass has been developed. The disadvantages of glass, or more precisely the method of its production, include the high energy intensity of obtaining the material, because glass is boiled for a long time at high temperatures.
Mica materials The main advantage of mica is its high heat resistance along with fairly high electrical insulating characteristics. In electrical engineering, two types of micas are used: muscovite KAl 2 (AlSi 3 O 10 )(OH) 2 and phlogopite KMg 3 (AlSi 3 O 10 (OH) 2. The high electrical insulating characteristics of mica are due to its unusual structure, namely, layering. Among others properties of mica, we note a low tg, less than; high resistivity, more than Ohm m; fairly high electrical strength, more than 100 kV/mm; heat resistance, melting point more than 1200 C.
Mica is used as electrical insulation, either in the form of plucked thin plates, incl. glued together (mikanites), and in the form of mica papers, incl. impregnated with various binders (mica or mica plastics). Mikanites have better mechanical characteristics and moisture resistance, but they are more expensive and less technologically advanced. Application: slot and turn insulation of electrical machines.
Mica are sheet materials made from mica paper based on muscovite. Sometimes they are combined with a substrate made of fiberglass (glass-ludinite) or polymer film (film-mudinite). Papers impregnated with varnish or other binders have better mechanical and electrical characteristics than unimpregnated papers, but their heat resistance is usually lower, because it is determined by the properties of the impregnating binder.
Mica plastics are sheet materials made from phlogopite-based mica paper and impregnated with binders. The use of mica and mica plastics is insulation of electrical machines, heat-resistant insulation of electrical devices. To conclude this section, I would like to provide comparative data on the electrical strength under normal conditions of some common gaseous, liquid and solid dielectrics.
Name of dielectric Air SF6 hydrogen helium C 14 F 24 transformer oil capacitor oil cable oil castor oil Polymethyl methacrylate Electric strength, kV/cm (50 Hz)
Name of dielectric Polyethylene mica polyvinyl chloride phenoplast electrical insulator porcelain (steatite) Electrocardboard (impregnated) Getinax Glass (quartz) Silicone rubber Fluoroplastic Electric strength, kV/cm (50 Hz) (70-160)