Sometimes it is necessary to connect the electrodes of a device or element to a DC power source. They are connected, observing polarity. Cathode and anode are the names of the conductors (electrodes) of the device with which this connection is made. There is no clear definition of these two terms. They are distinguished depending on the chemical and physical processes in which these designations are used.
Anode and cathode
Concept of cathode and anode
In electrical engineering, the terminal connected to the positive terminal of the power supply (PS) is called the anode (A). The electrode connected to the minus terminal of the IP is the cathode (K). Translated from Greek, anode means “ascending, upward movement,” and cathode means “descending, downward movement.” These names can be found in such sections of physics and chemistry as:
- galvanic power supplies;
- electrolysis and electroplating;
- semiconductors and vacuum electronics.
In addition, these terms denote the terminals of elements on the circuits and the signs of their charge.
The concept of cathode and anode - a simple explanation
In complex substances, electrons are distributed unequally between atoms in compounds. As a result of interaction, particles move from an atom of one substance to an atom of another. The reaction is called redox. The loss of electrons is called oxidation, the element that loses electrons is called a reducing agent.
The addition of electrons is called reduction; the receiving element in this process is the oxidizing agent. The transfer of electrons from a reducing agent to an oxidizing agent can occur through an external circuit, and then it can be used as a source of electrical energy. Devices in which the energy of a chemical reaction is converted into electrical energy are called galvanic cells.
The simplest classical example of a galvanic cell is two plates made of different metals and immersed in an electrolyte solution. In such a system, oxidation occurs on one metal and reduction occurs on another.
IMPORTANT! The electrode at which oxidation occurs is called the anode. The electrode on which reduction occurs is the cathode.
From school chemistry textbooks there is an example of a copper-zinc galvanic cell that operates using the energy of the reaction between zinc and copper sulfate. In the Jacobi-Daniel device, a copper plate is placed in a copper sulfate solution (copper electrode), and a zinc plate is immersed in a zinc sulfate solution (zinc electrode). The zinc electrode releases cations into the solution, creating an excess positive charge in it, while at the copper electrode the solution is depleted in cations, here the solution is negatively charged.
Designation in electrochemistry and non-ferrous metallurgy
Cathode - definition and practical application
The concept of anodes in electrolytic processes applies to positively charged electrodes. Electrolysis, by which various chemical elements are isolated or purified, is the effect of electric current on an electrolyte. Electrolytes are solutions of salts or acids. The other electrode involved in this reaction is the cathode.
Attention! A reduction reaction occurs at the negatively charged cathode (K), and an oxidation process occurs at the anode (A). In this case, “A” can be partially destroyed, participating in the purification of metals from unwanted additives.
In the metallurgical industry, anodes are used when applying protective layers to a product using the electrochemical method (plating) or electro-refining. Electrical cleaning allows you to dissolve rough metal (with impurities) on “A” and deposit it on “K” in a purified form.
A number of commonly used anodes are made of metals:
- zinc;
- copper;
- nickel;
- cadmium;
- lead (an alloy of lead and antimony);
- silver;
- gold;
- platinum.
Nickel plating, galvanizing and other application of protective or aesthetically sought-after coatings on products are carried out mainly from base metals.
With the help of “A” from precious metals, the electrical conductivity of components of electrical products is increased and layers of precious metals are applied to jewelry.
For your information. The pure metal deposited on the cathode is also called the "cathode". For example, pure copper obtained in this way is called “copper cathode”. Then it is used to make copper foil, wire and other things.
Metal refining
Other meanings of this word:
- "rising" electrode
- "Sidekick" of the cathode
- battery claw
- Battery "foot".
- "Magnet" in a diode
- "Morally stable" electrode
- "morally resistant" electrode
- "plus" radio tube
- Anagram for the word "one"
- anticathode
- Antipode of the cathode
- Battery vis-a-vis cathode
- Without it, the cathode is not a cathode
- Eternal vis-a-vis cathode
- vis-a-vis cathode
- Cathode vis-a-vis.
- Greek "way up"
- Greek "way up"
- Electrons run towards him
- Cathode and...
- A place for a “party” will be provided. particles
- The collection point will be set. particles
- positive particle collection site
- collection point for positive particles (electr.)
- A jumble of letters from the word “one”
- the name of this electrode comes from the Greek “way up”
- Partner and responder to the cathode
- cathode partner
- Cathode Electrolysis Partner
- Cathode partner.
- One of the electrode poles
- Birds of a feather with a cathode
- Cathode Opponent
- Opponent of the cathode.
- Electrons flee from it
- the movement of electrons is directed from it
- The movement of electrons is directed from it
- Pair to cathode
- Pair to the cathode.
- plus battery
- Pos. in all respects an electrode
- Positively charged electrode
- Positive “partner” of the cathode
- electrode positive in all respects
- positive end
- Positive contact
- positive pole of the current source
- Positive pole of the electric current source
- Positive electrode
- Battery positive electrode
- Positive electrode
- Battery pole
- Electrode pole
- Attracts anions
- Brother cathode
- Battery electrode with sign. "plus"
- Device electrode
- Electrode of a radio or electrical device
- Electrode with "+"
- Electrode with “high. moral qualities."
- Electrode with "plus"
- electrode with high moral qualities
- Electrode with plus
- electrode with a “+” sign
- Electrical device electrode
- Electrode "crusader"
- Electrode, “I don’t agree.” with cathode
- Electrode “disagreeing” with the cathode
- Crusader electrode
- Electropositivity
Anode and cathode in vacuum electronic devices
Characteristics of in5822 Schottky diodes
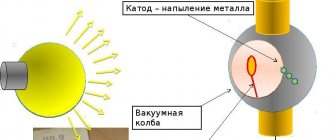
An electron tube is the simplest vacuum device. It consists of the following parts:
- cathode;
- grids;
- anode.
These three elements make up a vacuum diode. It has a cylindrical “K”, inside of which there is a filament. It heats up “K” to increase thermionic emission. In such devices, electrons leave “K” and travel to “A” in a vacuum, thereby creating an electric current. The anode is the electrode of the lamp with a positive potential. It is made in the form of a box surrounding the mesh and “K”. It can be made of molybdenum, tantalum, graphite, nickel. Its design is different, sometimes it has fins for heat removal.
The grid is an element located in the middle that controls the flow of particles. Most often it is made in the form of a spiral wrapping around the cathode.
Important! The larger the surface area of the cathode, and the hotter it is, the more current flows through the lamp.
“A” and “K” for a vacuum diode
Anode and cathode in semiconductor devices
What is a diode - operating principle and device
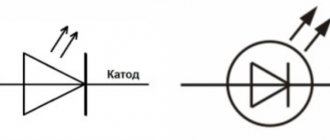
Semiconductor elements conduct electricity in a specific direction. If we consider a semiconductor diode, then its electrodes are also called “cathode” and “anode”. When a direct voltage is applied to it: a positive charge to the anode, the diode is open. If a positive potential comes to the cathode, the diode is closed. Such a diode has a pn junction between these two regions and is picky about the applied polarity. The output of an element from the p-region is called “A”, from the n-region - “K”.
Semiconductor diode
Anode and cathode sign
Which sign is indicated by “K”, which “A”, depends on what procedure and in what area is being considered. In electrochemistry, there are two devices that have different symbols: an electrolyzer and a galvanic cell.
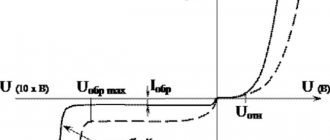
During electrolysis (redox chemical interaction under the influence of an external IP), the minus “-” denotes the cathode. It is on it that metals are reduced due to an excess of electrons. The plus “+”, in turn, marks the anode (positive electrode), where metals are oxidized due to a lack of negatively charged particles.
Signs of charges during electrolysis
In a galvanic cell, oxidation occurs without external influence of electricity. If we take a copper-zinc battery as an example, a large number of electrons (minus) accumulate at the anode. When moving along the external chain, they participate in the reduction of copper. This means that in this case the positive electrode will be the cathode.
Attention! In galvanic cells, the plus is the cathode, the minus is the anode. In electrolyzers, it’s the other way around—the anode is considered a plus, and the cathode is considered a minus.
Charge signs for a galvanic battery
For semiconductor devices, both the sign and the term are clearly assigned to the pins of the part. The anode is the “plus”, the cathode is the “minus” of the diode.
Exploring the battery
Low battery
During the discharge, the element voltage U, the difference between positive and negative, decreases (Fig. 2, 3).
- The potential of the positive electrode E+I≠0 becomes less than its value at rest E+I = 0 : E+I≠0 → the positive electrode is the cathode.
- The potential of the negative electrode EI≠0 becomes greater than its value at rest EI=0: EI>0 > EI=0 → the negative electrode is the anode.
Rice. 2: Battery discharge and charge: on the left - potential change in the positive and negative electrodes; on the right - change in battery voltage
Why is there confusion?
Everything happens because there is no clear connection between minus and plus to the components called “K” and “A”. Michael Faraday also came up with a simple polarity marking rule for this pair of electrodes. What is an anode, according to his explanation? When memorizing the definition, the scientist suggested drawing an analogy with the Sun. Where the current enters (rising) is the anode, where the current leaves (sunset) is the cathode. For batteries, the polarity at the anode and cathode changes depending on whether it operates as a galvanic cell (when discharging) or as an electrolyser (when charging).
DC welding also ambiguously determines “A” and “K” when igniting the arc with direct or reverse polarity.
Signs “A” and “K” for DC welding
How to determine anode and cathode
What a cathode and anode are is clarified in particular moments: when determining the terminals of semiconductor elements or when identifying electrodes in electrochemical processes.
A semiconductor diode requires positional placement in electrical circuits. For correct connection it is necessary to identify the pins. This can be done according to the following criteria:
- markings applied to the element body;
- length of part leads;
- tester readings when taking measurements in ohmmeter mode or checking diodes;
- using a current source with known polarity.
Marking of semiconductors of this type can be done by applying a graphic designation of a diode to the housing. Then minus (K) is the output from the side of the vertical line into which the arrow outline rests. The diode leg from which the arrow comes out is the plus (A). This is how the forward direction of the current is graphically indicated - from “A” to “K”.
Another way to designate the anode of a diode element can be one or two colored dots or a pair of narrow rings applied to the body. There are structurally designed diodes in which the negative (cathode) terminal is marked with a wide silver ring. The 2A546A-5 (DM) diode serves as such an example.
Examples of marking diodes
The length of the LED legs, which have never been soldered into boards, can also indicate the polarity of the leads. For LED diodes, the long leg is the positive electrode, and the short leg is the negative terminal. In addition, the shape of the body (the edge of the circle) can serve as a guide.
Polarity of LED diode terminals
When using a multimeter to determine the polarity of the contact terminals of a semiconductor, connect it in diode testing mode. If numbers appear on the display, it means the diode is connected in the forward direction. In this case, the red probe is connected to the anode “+”, the black one is connected to the cathode “-”.
If you don’t have a tester at hand, you can determine the names of the diode terminals by assembling a series circuit from a battery, a light bulb and a diode. When switched on directly, the light bulb will light up, which means that the batteries are plus on the anode and similarly minus on the other electrode.
Information. The LED electrodes can be identified using a constant voltage supply with a known polarity and a current-limiting resistor connected in series. The glow of the element will indicate direct switching. For this purpose, you can take a 3 volt RG2032 battery and a 1 kOhm resistor.
Turning on an LED via a limiting resistor
As for semiconductors, there is always a strict correspondence of names. In other cases, correct determination of the ongoing electrochemical reactions will help to clearly navigate the identification of electrodes.
Application in electrochemistry
In this branch of chemistry, a cathode is a negatively charged electrical conductor (electrode) that attracts positively charged ions (cations) during oxidation and reduction processes.
Electrolytic refining is the electrolysis of alloys and aqueous solutions. Most non-ferrous metals undergo this type of cleaning. Electrolytic refining produces metal with high purity. Thus, the purity of copper after refining reaches 99.99%.
Electrolysis of copper
An electrolytic process takes place on the positive electrical conductor during refining or purification. During this process, metal with impurities is placed in an electrolyzer and made into an anode. Such processes are carried out using an external source of electrical energy and are called electrolysis reactions. They are carried out in electrolyzers. It functions as an electric pump, pumping negatively charged particles (electrons) into the negative conductor and removing it from the anode. It doesn't matter where the current comes from.
At the cathode, the metal is cleaned of foreign impurities. A simple cathode is made of tungsten, sometimes tantalum. The advantage of a tungsten negative electrode is the durability of its manufacture. Disadvantages include low efficiency and cost-effectiveness. Complex cathodes have different structures. Many of these types of conductors have a special layer applied to the bare metal on top, which enables greater performance at relatively low temperatures. They are very economical. Their disadvantage is that their performance is not very stable.
The finished pure metal is also called a cathode. For example, a zinc or platinum cathode. In production, the negative conductor is separated from the cathode base using cathode stripping machines.
When negatively charged particles are removed from an electrical conductor, an anode is created on it, and when negatively charged particles are pumped onto an electrical conductor, a cathode is created. During electrolysis of the metal being purified, its positive ions attract negatively charged particles on the negative conductor, and a reduction process occurs. The most commonly used anodes are:
- zinc;
- cadmium;
- copper;
- nickel;
- tin;
- gold;
- silver;
- platinum.
Zinc anodes are most often used in production. They are:
- rolled;
- cast;
- spherical.
Rolled zinc anodes are most commonly used. Nickel and copper are also used. But cadmium is almost never used because of its toxicity to the environment. Bronze and tin anodes are used in the manufacture of electronic printed circuit boards.
Galvanization (galvanostegy) is the process of applying a thin layer of metal to another object in order to prevent corrosion of the product, oxidation of contacts in electronics, wear resistance, and decoration. The essence of the process is the same as during refining.
Zinc and tin are used to increase the corrosion resistance of the product. Galvanizing can be cold, hot, galvanic, gas-thermal and thermal diffusion. Gold is used mainly for protective and decorative purposes. Silver increases the resistance of electrical appliance contacts to oxidation. Chrome – to increase wear resistance and protect against corrosion. Chrome plating gives products a beautiful and expensive look. Used for applying to handles, taps, rims, etc. The chrome plating process is toxic, therefore it is strictly regulated by the legislation of different countries. The picture below shows the galvanization method using nickel.
Nickel plating of a kettle using galvanization
Video
Coffee capsule Nescafe Dolce Gusto Cappuccino, 3 packs of 16 capsules
1305 ₽ More details
Coffee capsules Nescafe Dolce Gusto Cappuccino, 8 servings (16 capsules)
435 ₽ More details
Stabilizers for smartphones