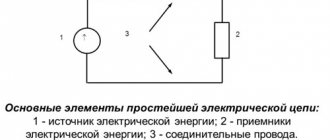
Providing the needs of humanity with sufficient energy is one of the key tasks facing modern science. In connection with the increase in energy consumption of processes aimed at maintaining the basic conditions of society, acute problems arise not only in the generation of large volumes of energy, but also in the balanced organization of its distribution systems. And the topic of energy transformation is of key importance in this context. The coefficient of production of useful energy potential, as well as the level of costs for servicing technological operations within the framework of the infrastructure used, depend on this process.
General information about conversion technology
You may be interested in: Roast is... Interpretation and synonyms
The need to use different types of energy is associated with differences in the processes that require a power resource. Heat is required for heating, mechanical energy is required to power the movement of mechanisms, and light is required for lighting. Electricity can be called a universal source of energy both in terms of its transformation and in terms of its application in various fields. Natural phenomena are usually used as initial energy, as well as artificially organized processes that contribute to the generation of the same heat or mechanical force. In each case, a certain type of equipment or complex technological structure is required, which, in principle, allows for the conversion of energy into the form required for final or intermediate consumption. Moreover, among the tasks of the converter, not only transformation stands out as the transfer of energy from one type to another. Often this process also serves to change some energy parameters without transforming it.
You may be interested in: Pavel Pavlovich Demidov: charity, family and career
The transformation itself can be single-stage or multi-stage. In addition, for example, the operation of photocrystalline solar generators is usually considered as the transformation of light energy into electricity. But at the same time, it is also possible to transform the thermal energy that the Sun gives to the soil as a result of heating. Geothermal modules are placed at a certain depth in the ground and, through special conductors, fill batteries with energy reserves. In a simple conversion scheme, the geothermal system provides the accumulation of heat energy, which is given to the heating equipment in its pure form with basic preparation. The complex structure uses a heat pump in a single group with heat condensers and compressors that provide heat conversion and electricity.
Potential energy
Via GIPHY
The potential energy of a body also depends on the mass of the object.
Potential energy is another basic type of energy and is related to the position or state of an object in relation to another .
Potential energy increases when attracted bodies separate or when thrown or repelled bodies come together. The region in which objects are attracted or repelled is called a force field . Examples of force fields can be, for example, the Earth's gravitational force field or a magnetic force field.
Potential and kinetic energy
Potential energy is converted into kinetic energy and can also be found in other forms of energy such as gravitational potential energy or elastic potential energy.
Gravitational potential energy
At the moment when an athlete reaches the highest point, he has more potential energy.
When potential energy is related to the gravitational force, it is called gravitational potential energy . The gravitational force field around our planet pulls objects toward its center. When we lift objects away from the Earth, we increase their gravitational potential energy.
There is potential gravitational energy between the Sun and the planets, and between the Moon and Earth. In fact, tides are the result of the gravitational pull that the Moon creates on Earth's bodies of water.
Elastic potential energy
Via GIPHY
When we stretch a spring, the energy to return to its original shape is stored as potential energy.
Another form of potential energy is the energy that a spring contains when we stretch or compress it. This energy is called elastic potential energy : it is the energy of materials when they are stretched or twisted. When we compress a spring, we increase its potential energy.
Elastic potential energy is what moves the spring. Also in pole vaulting in track and field we have an example of elastic potential energy being converted into gravitational potential energy.
Types of electrical energy conversion
You might be interested in:Matrixes: Gaussian method. Matrix calculation using the Gaussian method: examples
There are different technological methods for extracting primary energy from natural phenomena. But even more opportunities for changing the properties and forms of energy are provided by accumulated energy resources, since they are stored in a form convenient for transformation. The most common forms of energy conversion include radiation, heating, mechanical and chemical operations. The most complex systems involve molecular degradation processes and multilevel chemical reactions that combine multiple transformation steps.
The choice of a specific transformation method will depend on the conditions of the process organization, the type of initial and final energy. Among the most common types of energy that, in principle, participate in transformation processes are radiant, mechanical, thermal, electrical and chemical energy. At a minimum, these resources are successfully exploited in industry and households. Indirect energy conversion processes, which are derivatives of one or another technological operation, deserve special attention. For example, metallurgical production requires heating and cooling operations, which produce steam and heat as derived, but not target, resources. In essence, these are waste products that are also used, transformed or used within the same enterprise.
Solar energy
The sun is the most important source of energy for life on Earth.
Solar energy is the radiant energy of the sun . It travels through space until it reaches Earth in the form of electromagnetic waves. Most of the solar radiation that reaches the Earth's atmosphere is ultraviolet radiation, visible light, and infrared rays.
The sun is made of hydrogen and helium. In this case, the energy comes from the process of nuclear fusion: hydrogen nuclei combine to form helium and radiant energy.
People have learned to use solar energy. Today, the energy of sunlight is used to heat houses and buildings, increasing their thermal energy. Visible sunlight passes through glass windows and is absorbed by materials inside the room. This causes the materials to heat up.
The radiant energy of the Sun is responsible for the existence of life on Earth. Plants harvest this energy to produce food, converting it into chemical energy. Solar energy controls the movement of air in the atmosphere, causing winds.
Heat energy conversion
One of the oldest energy sources in terms of development and the most important for maintaining human life, without which it is impossible to imagine the life of modern society. In most cases, heat is converted into electricity, and a simple scheme for such transformation does not require connecting intermediate stages. However, in thermal and nuclear power plants, depending on their operating conditions, a preparation stage with the conversion of thermal into mechanical energy can be used, which requires additional costs. Today, direct thermoelectric generators are increasingly used to convert thermal energy into electricity.
You may be interested in: Maxwell's Law. Maxwell's velocity distribution
The transformation process itself occurs in a special substance, which is burned, releases heat and subsequently acts as a source of current generation. That is, thermoelectric installations can be considered as zero-cycle sources of electricity, since their operation starts even before the appearance of base thermal energy. The main resource is fuel elements – usually gas mixtures. They are burned, resulting in heating of the heat-distributing metal plate. In the process of heat removal through a special generator module with semiconductor materials, energy conversion occurs. Electric current is generated by a radiator unit connected to a transformer or battery. In the first option, energy is immediately supplied to the consumer in finished form, and in the second, it is accumulated and released as needed.
Energy in food
Everything you do every day, even sleeping, requires energy. Different activities require different amounts.
How much energy does your body need every day:
Male or female, young or old, active or not, people get the resources they need every day from the food they eat. This food is a form of chemical potential energy. When food is used up in the cells of the body during respiration, chemical potential energy is released. Different products allocate different amounts of resources.
Daily energy needs of women and men in (kilojoules)
| Age | Women (kilojoules) | Men (kilojoules) |
| 5 years | 7000 | 7000 |
| 10 years | 9000 | 10000 |
| 15 years | 9500 | 13000 |
| 20 years | 9500 | 12500 |
| 25 year olds | 9000 | 11500 |
Keep yourself healthy without being overweight — — — — > balance your intake with your expenditure
Some types of food provide more energy than others. Fats provide twice as much as carbohydrates. Since fat provides more than other types of food, you might think that food is always good for us. THIS IS WRONG! The body can only use so much food at once. He uses everything he needs and stores the excess as fat. This can lead to obesity and other health problems.
When you are active, your body burns a lot of energy. When you watch TV or play on the computer, your body burns much less.
More of the energy our bodies receive from food is converted into heat through respiration. This is used to keep our bodies at a certain temperature constantly (37 degrees C). This is important if the chemical reactions that occur in cells are to work efficiently.
To find out how much energy is stored in food, you can turn it into heat and measure what that heat can do.
Generation of thermal energy from mechanical
Also one of the most common ways to obtain energy as a result of transformation. Its essence lies in the ability of bodies to give off thermal energy in the process of doing work. In its simplest form, this energy transformation scheme is demonstrated by the example of the friction of two wooden objects, which results in fire. However, to use this principle with tangible practical benefit, special devices are required.
In households, the transformation of mechanical energy takes place in heating and water supply systems. These are complex technical structures with a magnetic core and a laminated core connected to closed electrically conductive circuits. Also inside the working chamber of this design there are heating pipes, which are heated under the action of the work done from the drive. The disadvantage of this solution is the need to connect the system to the electrical network.
In industry, more powerful liquid-cooled converters are used. The source of mechanical work is connected to closed water tanks. During the movement of the executive bodies (turbines, blades or other structural elements) conditions for vortex formation are created inside the circuit. This happens during moments of sudden braking of the blades. In addition to heating, in this case the pressure also increases, which facilitates the processes of water circulation.
Sound energy
Via GIPHY
The bell vibrates when struck and produces sound waves that travel through the air.
Sound energy is the mechanical energy of particles that vibrate in the form of waves through a transmission medium . The medium through which sound waves travel can be air, water or other materials. Anything that causes noise generates sound energy.
Sound travels faster in solids than in liquids and faster in liquids than in gases. Therefore, if you put your ear to the floor, you can hear, because the speed of sound on the ground is four times higher than in the air.
It is thanks to sound energy that we can hear. When sound waves in the air enter your ears, they stimulate special cells that send information to the brain. The more energy a sound wave has, the louder the sound will be.
The seabed maps are made using a sound system. The sonar sends out sound waves and calculates the distance traveled using the speed of sound in water.
In medicine, ultrasound is used to remove kidney stones. An echocardiogram is another technology that uses sound waves to see the fetus in pregnant women.
Conversion of electromechanical energy
Most modern technical units operate on the principles of electromechanics. Synchronous and asynchronous electrical machines and generators are used in transport, machine tools, industrial engineering units and other power plants for various purposes. That is, electromechanical types of energy conversion are applicable to both generator and motor operating modes, depending on the current requirements of the drive system.
In a generalized form, any electrical machine can be considered as a system of mutually moving magnetically coupled electrical circuits. Similar phenomena also include hysteresis, saturation, higher harmonics and magnetic losses. But in the classical view, they can be classified as analogues of electric machines only if we are talking about dynamic modes when the system operates within the energy infrastructure.
The electromechanical energy conversion system is based on the principle of two reactions with two-phase and three-phase components, as well as the method of rotating magnetic fields. The rotor and stator of motors perform mechanical work under the influence of a magnetic field. Depending on the direction of movement of charged particles, the operating mode is set - as a motor or generator.
Energy in everyday life
Everything around us depends on energy. Cars depend on the fuel stored in them to use. Used in homes, offices and industry to make all types of machines work. It is used to light and heat our homes, to cook and store our food.
Obviously, it is difficult to say what energy is, but it is important to us. It's easier to say what she's capable of.
Energy is the ability to do work.
Everything that works must have a supply of energy. The motorcycle will not continue to run if it is not supplied with gasoline. Gasoline provides the resources that the engine uses to operate.
When a person pedals a bicycle, the force comes from the muscles in your body. Your muscles get energy from the food you eat. If more is needed than a person has, then the emergency energy is stored in the body as fat. On the other hand, an inadequate energy diet will lead to a thin and truly unhealthy body!
Everything we do requires energy, even sleep! The table below shows the quantities required for various activities.
Energy involved in daily activities:
| Activity: | kJ per hour: |
| Man sleeps | 200 |
| Sitting at a meeting | 300 |
| Easy job | 550 |
| Walking | 700 |
| Active work | 850 |
| Goes uphill | 1000 |
| Cycling | 1150 |
| Run | 1700 |
| Electrical energy used by the average household by the entire family, per day | 80,000 kJ |
Generating electricity from chemical energy
The total chemical source of energy is traditional, but methods of its transformation are not so common due to environmental restrictions. Chemical energy itself is practically never used in its pure form, at least not in the form of concentrated reactions. At the same time, natural chemical processes surround a person everywhere in the form of high- or low-energy combinations, which manifest themselves, for example, during combustion with the release of heat. However, the conversion of chemical energy is purposefully organized in some industries. Typically, conditions are created for high-tech combustion in plasma generators or gas turbines. A typical reagent for these processes is a fuel cell, which contributes to the production of electrical energy. From an efficiency point of view, such transformations are not as beneficial as compared to alternative methods of generating electricity, since some of the useful heat is dissipated even in modern plasma installations.
Magnetic energy
Magnets are used to grip magnetic materials such as nuts and bolts.
The ability of an object to do work due to its position in a magnetic field is the magnetic field's . Magnets have a magnetic field and two regions called magnetic poles. Equal poles are rejected and unlike poles are attracted. The most commonly used magnetic materials are iron and its alloys.
For example, an iron screw that approaches a magnet but does not touch it has magnetic potential energy. Objects move in a direction that reduces their magnetic potential energy.
Microphones, for example, work well thanks to magnetic energy. The operation is as follows: the microphone has a membrane that vibrates with sound. This vibration is transmitted to a cable wrapped around a magnet, which sends an electrical signal to the amplifier, making the sound louder. In this case we have the conversion of sound energy into magnetic energy, then electrical energy and then sound energy.
Electromagnetic levitation railways are another example of how we can use magnetic energy to do work. The railroad moves through a magnetic field that moves along a ferromagnetic path.
Conversion of solar radiation energy
As a method of energy conversion, the process of processing sunlight may in the near future become the most popular in the energy sector. This is due to the fact that even today every homeowner can theoretically purchase equipment for converting solar energy into electrical energy. The key feature of this process is that the accumulated sunlight is free. Another thing is that this does not make the process completely cost-free. Firstly, there will be costs associated with the maintenance of solar batteries. Secondly, generators of this type themselves are not cheap, so few people can yet afford the initial investment in organizing their own mini-power station.
You will be interested in: Stepan Pavlovich Suprun (Soviet test pilot, military fighter pilot): biography, history of death, awards, memory
What is a solar energy generator? This is a set of photovoltaic panels that convert the energy of the sun's rays into electricity. The very principle of this process is in many ways similar to the operation of a transistor. Silicon is used as the main material for the manufacture of solar cells in different versions. For example, a device for converting solar energy can be poly- or monocrystalline. The second option is preferable in terms of performance characteristics, but is more expensive. In both cases, the photocell is illuminated, during which the electrodes are activated and, in the process of their movement, an electrodynamic force is generated.
Radiant Energy
Light is radiant energy that travels in waves.
Energy in the form of light or heat is radiant energy, more commonly known as radiation. Radiation is electromagnetic waves that do not require a means of travel like sound waves to travel through space. The source of electromagnetic waves are electrons that vibrate, creating an electric field and a magnetic field.
The different types of radiant energy or radiation (fluxes) are ordered by energy levels in the electromagnetic spectrum. They travel through space at a speed of 300 million meters per second, that is, the speed of light.
X-rays and gamma rays are invisible radiations with a lot of energy. Both have important medical applications. X-rays are used to diagnose bone fractures, while gamma rays are used to diagnose neurological diseases such as Parkinson's and Alzheimer's, or heart disease.
Ultraviolet (UV) rays are a type of invisible radiation created by the Sun and some special lamps. These rays are responsible for the tan we get when we expose ourselves to the sun. However, overexposure to ultraviolet rays can cause burns and skin cancer. That's why you should protect your body when you're out in the sun for long periods of time, especially your skin (to protect against skin cancer) and eyes.
Visible light radiation is what the human eye can perceive. Usually we see white light, which is nothing more than a mixture of lights of different colors. Light comes in packets of energy called photons, which have no mass.
Infrared radiation, microwaves, and radio waves are less energetic radiation on the electromagnetic spectrum. Radio waves and microwaves are waves used in communications to transmit sound and images.
Steam energy conversion
Steam turbines can be used in industry as a way to transform energy into an acceptable form, and as an independent generator of electricity or heat from specially directed flows of conventional gas. Turbine machines are not the only ones used as devices for converting electrical energy in conjunction with steam generators, but their design is optimally suited for organizing this process with high efficiency. The simplest technical solution is a turbine with blades, to which nozzles with supplied steam are connected. As the blades move, the electromagnetic installation inside the apparatus rotates, mechanical work is performed and current is generated.
Some turbine designs have special extensions in the form of stages, where the mechanical energy of the steam is converted into kinetic energy. This feature of the device is determined not so much by the interests of increasing the energy conversion productivity of the generator or by the need to generate kinetic potential, but by providing the possibility of flexible regulation of the turbine operation. The expansion in the turbine provides a control function, which makes it possible to effectively and safely regulate the amount of energy generated. By the way, the expansion working area, which is included in the conversion process, is called the active pressure stage.
Description of convection
Convection is another method of heat transfer. Its essence lies in the transfer of internal energy through layers of liquid or gaseous substances.
Since convection occurs only when substances move, such a process can only occur in liquids and gases. It is known that physical bodies in these two states conduct heat poorly, but thanks to the concept they can still be heated. The effective use of this process can be observed in the cold season, when the air is warmed in rooms equipped with steam heating batteries. This type of heat transfer can be observed in a simple experiment:
- A crystal of potassium permanganate is carefully lowered to the bottom of a flask filled with water.
- The container is heated in the place where the permanganate salt is located.
- After some time, colored streams of water begin to rise from the bottom.
- Having risen to the upper layers, the jets descend.
The lower layer of liquid expands when heated, which leads to an increase in its volume and a decrease in density. Under the influence of the Archimedean force, the heated part of the substance moves higher. Cold liquid is dropped onto the vacated space, which rises as it heats up. In this case, internal energy is transferred by upward moving water flows.
Heat transfer occurs in gases in a similar way. So, if a paper pinwheel is placed over a heat source, it begins to rotate. The blades of the object begin to move because the least dense layers of heated air rise due to the influence of the buoyant force on them, while at the same time the cold layers descend, taking the place of warm ones. This movement of air causes the pinwheel to rotate.
Methods of energy transfer
Methods of energy transformation cannot be considered without the concept of its transmission. Today, there are four ways of interaction of bodies in which energy transfer occurs - electrical, gravitational, nuclear and weak. Transmission in this context can also be considered as a method of exchange, therefore, the performance of work during energy transfer and the heat exchange function are fundamentally separated. What energy transformations involve doing work? A typical example is a mechanical force in which macroscopic bodies or individual particles of bodies move in space. In addition to mechanical force, magnetic and electrical work are also distinguished. The key unifying property for almost all types of work is the ability for complete quantitative transformation between themselves. That is, electricity is transformed into mechanical energy, mechanical work into magnetic potential, etc. Heat exchange is also a common method of energy transfer. It can be undirected or chaotic, but in any case there is movement of microscopic particles. The number of activated particles will determine the amount of heat - useful heat.
Mechanical energy
Mechanical energy is the sum of position and motion energy.
The mechanical energy of a body covers the movement and position of an object, that is, it is the sum of the kinetic and potential energy of that object.
When we swing, we convert kinetic energy into potential and vice versa, so we can move faster and higher.
For example, the child on the skateboard in the previous image has kinetic energy that allows him to anchor himself to the wall, gaining potential energy. When it starts to fall, the potential energy turns into kinetic energy and picks up speed.
Convection
Answering the question of what heat transfer is, let’s consider the process of heat transfer in liquids or gases through spontaneous or forced mixing. In the case of forced convection, the movement of matter is caused by the influence of external forces: fan blades, pump. A similar option is used in situations where natural convection is not effective.
A natural process is observed in cases where, due to uneven heating, the lower layers of the substance are heated. Their density decreases and they rise upward. The upper layers, on the contrary, cool, become heavier, and sink down. Then the process is repeated several times, and with mixing, self-organization into a structure of vortices is observed, and a regular lattice is formed from convection cells.
Thanks to natural convection, clouds form, precipitation occurs, and tectonic plates move. It is through convection in the Sun that granules are formed.
Proper use of heat transfer ensures minimum heat loss, maximum consumption.
Heat capacity
Different substances have different abilities to store heat; this depends on their molecular structure and density. The amount of heat required to raise the temperature of a unit mass of a substance by one degree (1 °C or 1 K) is called its specific heat capacity. Heat capacity is measured in J/(kg•K).
Typically, a distinction is made between heat capacity at constant volume (CV) and heat capacity at constant pressure (CP), if during the heating process the volume of the body or pressure, respectively, is maintained constant. For example, to heat one gram of air in a balloon by 1 K, more heat is required than for the same heating in a sealed vessel with rigid walls, since part of the energy imparted to the balloon is spent on expanding the air, and not on heating it. When heated at constant pressure, part of the heat is used to produce the work of expansion of the body, and part is used to increase its internal energy, while when heated at constant volume, all the heat is spent on increasing internal energy; in this regard, CP is always greater than CV. For liquids and solids, the difference between CP and CV is relatively small.
We follow the molecules of an ideal gas
Some properties of ideal gas molecules can be studied as if these molecules were racing like cars around you. For example, the average kinetic energy for each molecule can be calculated using a very simple formula:
where \( k \) is Boltzmann’s constant, equal to 1.38·10-23 J/K, and \( T \) is the temperature. And since you can get the mass of each molecule if you know for which gas the calculation is being carried out (see above), you can calculate the speeds of the molecules at different temperatures.
Calculating the speed of air molecules
Imagine that one fine spring day you are on a picnic with friends. You have a wonderful meal: potato salad, sandwiches and drinks. But after a while you remember about physics, fall on your back and start looking at the sky. Physics at a picnic, what could be more boring? No way. Physics is present everywhere: in any place and at any time, even if direct signs of its presence are not at all obvious.
Even if you can't see the air molecules scurrying around, using the laws of physics you can easily calculate their average speed. All you need is a calculator and a thermometer. Let's assume that the measured air temperature turned out to be approximately 28°C, or 301 K (you can learn how to convert Celsius and Kelvin into each other in Chapter 13). As is known, the average kinetic energy of molecules in the air can be calculated using the formula:
It remains to substitute numerical values into it:
So, the “average” molecule has a kinetic energy of 6.23·10-21 J. However, molecules are very small - so what speeds will correspond to this value? As you can find out in Chapter 8:
where \( m \) and \( v \) are, respectively, mass and speed, then:
Air mainly consists of nitrogen molecules N2 (about 78%) and oxygen molecules O2 (about 21%). Without much loss of precision, let us assume that the air consists mainly of nitrogen molecules. A nitrogen molecule has a mass approximately equal to 4.65 10-26 kg (which you can calculate yourself by knowing the molecular mass of nitrogen and then dividing it by the number \(N_A\)). Substituting numbers into the last formula, we get:
Wow! Just imagine that such a huge number of “kids” crash into you every second at a speed of 1861 km/h! It's good that the molecules are so small. Imagine if each air molecule weighed about a kilogram.
Calculating the internal energy of an ideal gas
Atoms and molecules have very little mass, but there are a lot of them in gases, and since they all have kinetic energy, it is possible to determine their total kinetic energy or that part of the internal energy of a gas that consists of the energy of motion of its molecules. So, what kinetic energy does a given amount of gas have? Each molecule has an average kinetic energy:
To get the total kinetic energy, you need to multiply the average kinetic energy by the number of molecules present, equal to \( nN_A \), where \( n \) is the number of moles:
Here \(N_Аk\) equals \(R\), i.e. universal gas constant (see earlier in this chapter), so the previous formula takes the form:
So, 600 moles of helium at a temperature of 27°C have the following internal energy, which is associated with the thermal movement of molecules:
That's a little more than half a kilocalorie! This kind of unit of measurement of the conditional energy value of food (kcal) can be found on their packaging.
Chapter 15. Thermal energy and work: principles of thermodynamics →
← Chapter 13. An unexpected explanation of heat using thermodynamics
Chapter 14. Transfer of thermal energy in solids and gases
3 (60%) 3 votes
We derive the ideal gas law
The description of the behavior of gaseous states as required by physics begins when we move to the level of atoms and molecules. How is the concept of “mole” used to describe physical processes when gases are heated? It turns out that the behavior of different gases can be related to each other using physical concepts already known to us, such as moles, temperature, pressure and volume. This relationship is not entirely accurate for real gases in nature, but it describes the behavior of ideal gases very well. (An ideal gas is a gas in which the interaction of molecules is reduced to pair collisions, and the time of an intermolecular collision is much less than the average time between collisions. - Ed.) However, some real gases, for example helium, are described with very good accuracy as ideal, and They form a reliable experimental “stronghold” of thermodynamics.
It has been experimentally proven that if a gas is heated while keeping its volume constant, then, as shown in Fig. 14.5, the gas pressure will increase linearly. In other words, at constant volume:
where \( T \) is the temperature measured in kelvins, and \( P \) is the pressure.
If you change the volume, you will notice that the pressure will be inversely proportional to it, i.e. When the volume of a gas doubles, the pressure of this gas decreases by half:
On the other hand, when the volume and temperature of an ideal gas are constant, the pressure is proportional to the number of moles of gas present—when the amount of gas is doubled, the pressure doubles. If the number of moles is \( n \), then:
By inserting the constant \( R \) into the formula (the so-called universal gas constant, the value of which is 8.31 J/(mol K), we obtain the ideal gas law relating pressure, volume, number of moles and temperature to each other:
Gases for which this ideal gas law is satisfied are considered truly ideal. Using this law, you can predict the pressure of an ideal gas if you know its quantity, temperature and volume occupied by it.
The ideal gas law can be expressed slightly differently if we use Avogadro's number \( N_A \) (see previous section) and the total number of molecules \( N \):
The ratio \( R/N_A \) is also called the Boltzmann constant \( k \) and is equal to 1.38·10-23 J/K. Using this constant, the ideal gas law takes the following form:
Pressure: an example of using the ideal gas law
Let's assume that there is a tank with a volume of 1 m3 filled with 600 moles of helium (very close to an ideal gas) at a room temperature of 27°C. What will the gas pressure be? Using the following form of the ideal gas equation:
We get the following formula into which we substitute the numerical values:
The pressure in all walls of the vessel is 1.50 106 N/m2. Please note the pressure unit used, N/m2. This unit of measurement is used so widely that it has its own name in the SI system - pascal, or Pa. Atmospheric pressure is 1.013·105 Pa. In addition, pressure of one atmosphere is sometimes indicated in units of torr and 1 atmosphere = 760 torr. And in our example, the pressure is 1.50·106 Pa, or approximately 15 atmospheres.
Sometimes you have to deal with a special set of conditions that apply to gases, called normal conditions (or n.s.). They correspond to the following physical conditions: pressure is 1 atmosphere, i.e. 1.013·105 Pa, and the temperature is 0°C. Using the ideal gas law, it can be calculated that under normal conditions, 1 mole of an ideal gas occupies a volume of 2.24136·10-2 m3, or about 22.4 liters (1 liter is equal to 1·10-3 m3).
Boyle-Mariotte's law and Charles's law: alternative formulations of the ideal gas law
The ideal gas law is often formulated in different ways. For example, one can express the relationship between the pressure and volume of an ideal gas before and after one of these quantities changes at a constant temperature:
From this formula, expressing the Boyle-Marriott law, it follows that, other conditions remaining unchanged, the product \( PV \) will be preserved.
Further, if the pressure is constant, then we can say that:
From this formula expressing Charles’s law, it follows that for an ideal gas, under other constant conditions, the ratio \( V/T \) will be preserved.
Similarly, if the volume is constant, then we can say that:
From this formula expressing the Gay-Lussac law, it follows that for an ideal gas, under other constant conditions, the ratio \( P/T \) will be preserved.
Water and air pressure measurement
Let's try to apply our knowledge about pressure in everyday life. Well, the pressure under a layer of liquid, such as water, can be determined using a simple formula:
P = pgh.
Here p is the density of the liquid, g is the acceleration of gravity under the influence of gravity (9.8 m/s2), h is the depth for which the pressure is calculated. For water, p is equal to 1000 kg/m2, so it turns out:
P = (10000.0)(9.8)h = 9800h.
For every meter of depth to which you go underwater, the pressure increases by about 9800 Pa, or about 1/10 of an atmosphere (how to convert atmospheres and pascals into each other was described above).
This formula is correct as long as the density of the liquid does not change, i.e. For air, it is difficult to predict such pressure, because the density of air, as is known, varies right up to the airless space of outer space. There are often problems in physics that ask how much the air pressure will change if you rise to a particular height. In other words, in these problems it is proposed to find the pressure difference for a given height difference at which p is constant.
Understanding Avogadro's number
This chapter mainly deals with the action of thermal energy on solids and liquids (for example, in vessels with liquid metal), but a lot can be learned here about the processes of heating gases. First, let's figure out how many molecules we have to deal with. To do this, we don't need too much knowledge of physics. Imagine that someone found a large diamond and brought it to you for evaluation.
“How many atoms are there in my diamond?”
“It depends on how many moles are in it,” you answer.
The person, offended, says: “I ask you not to express yourself!”
A mole is the number of atoms in 12 grams of isotope 12 carbon. Isotope 12 carbon (commoned as 12C and also called carbon-12, or simply carbon 12) is the most common variant of carbon, although some carbon atoms have slightly more neutrons (in carbon-13 for sure), so on average there are about 12.011 of them. Before you know how many atoms you have, find out how many moles of a substance you have, and when working with gases you often need to know the number of atoms.
As a result of measurements, it was found that the number of atoms in a mole (the so-called Avogadro number, \( N_A \)) is equal to 6.022·1023. So now you know how many atoms are in 12 g of carbon-12. Will there be the same number of atoms in, say, 12 g of sulfur? In no case. Each sulfur atom is different in weight from each carbon atom, so even the same number of grams of each will have a different number of atoms.
How much more mass does sulfur have than carbon-12? By studying the periodic table hanging on the wall of the physics laboratory, you will learn that the atomic mass of sulfur is 32.06 (usually this is the number located to the right and below the symbol of the element, for example, for sulfur this symbol is S). But what exactly is 32.06? This refers to 32.06 atomic mass units (or amu), each equal to 1/12 the mass of a carbon-12 atom. Then if the mass of a mole of carbon-12 is equal to 12 g and the mass of the average sulfur atom is greater than the mass of a carbon-12 atom in this ratio:
then a mole of sulfur atoms should have the following mass:
How convenient! Knowing that a mole of an element has the same mass in grams as the atomic mass of that element in atomic mass units will still be useful in your calculations. The atomic mass of any element in atomic mass units can be found from any periodic table. For example, the mass of a mole of silicon is 28.09 g, a mole of sodium is 22.99 g, etc. And each of these moles contains 6.022·1023 atoms.
You will now be able to determine the number of atoms in diamond, which is one of the solid states of carbon (with an atomic mass of 12.01 amu). 12.01 g of diamond is 1 mole, so to calculate the number of atoms in a diamond, you need to determine the number of moles in a diamond and multiply this value of atoms in a mole, i.e. by 6.022·1023 atoms.
Not all bodies are composed of atoms of the same type. Most materials are composites, such as water, which has two hydrogen atoms (H2O) for every oxygen atom. In such cases, molecular mass, which is also based on atomic mass units, should be used instead of the atomic mass unit. For example, the molecular mass of water is 18.0153 amu, so the mass of one mole of water molecules is 18.0153 g.
We emit and absorb light: thermal radiation
How to quickly warm up after coming home in late autumn in wet and rainy weather? To a certain extent, we cannot do without the participation of physics here. Of course, you immediately want to take a warm bath! You can warm up not only with warm water, but even with the help of an incandescent lamp hanging in the bathroom (Fig. 14.4). Indeed, an incandescent lamp emits thermal energy and keeps you from freezing.
Thermal radiation is light that can transmit thermal energy. We receive thermal energy transmitted through radiation every day in the form of daylight. Indeed, the Sun is a huge thermal reactor located at a distance of 150 million km from us, and the thermal energy coming from it through the vacuum of space reaches the Earth without using thermal conduction or convection (see the two previous sections of this chapter). On Earth, solar energy is produced through radiation, which you can verify for yourself by simply standing outside on a clear day and exposing your face to the sun's rays. However, there is another way to get noticeable signs of radiation, namely to get a sunburn, which you will then have to deal with.
Thermal radiation: not visible, but felt
Any physical body around you is a source of constant thermal radiation, unless it has a temperature of absolute zero, which is unlikely, since it is physically very difficult to reach this temperature. For example, a serving of ice cream also emits thermal radiation. The fact is that thermal radiation is nothing more than electromagnetic radiation. Electromagnetic radiation occurs due to the acceleration and deceleration of electrical charges. At the molecular level, this is exactly what happens when physical bodies are heated: atoms move with acceleration and interact quite strongly with each other.
Even our bodies constantly emit energy, but this electromagnetic radiation is usually not visible, as it is in the infrared part of the spectrum. However, this light is visible with infrared devices, which are often referred to as “night vision devices.” We emit thermal energy constantly in all directions, and all objects around us also constantly emit thermal energy in all directions. If you and the environment around you are at the same temperature, then both you and this environment radiate energy towards each other with the same intensity.
When the environment does not radiate enough thermal energy towards you, a feeling of cold occurs. This is why space is considered so “cold”. But there is nothing cold to the touch, and thermal energy in space is not lost due to conduction or convection. The only thing that happens is that the space environment weakly radiates towards you, and you, thanks to your own radiation, will constantly lose thermal energy and very quickly begin to freeze.
When a body is heated to about 1000 K, it begins to shine with red light (this may explain why you, even when emitting, do not shine with red light from the visible part of the spectrum). And when a body gets hotter, its radiation shifts through the orange, yellow and other parts of the spectrum towards its white part, which is reached somewhere at 1700 K.
Heaters with a red-hot coil transfer thermal energy using thermal radiation. As for convection, it occurs when the air is heated, rises and spreads throughout the room (and the transfer of thermal energy through conduction occurs when you mistakenly touch the hot part of the heater, which is hardly a good example of heat transfer). However, from a heater with an incandescent coil, the transfer of thermal energy occurs mainly through radiation. Many homes today have heating wires built into the walls, ceilings or floors called radiant heating heaters. According to the architect's plan, these heaters are not visible, but if you stand facing one of them, you will immediately feel the heat caused by thermal radiation.
People intuitively understand the emission and absorption of thermal energy in the environment. For example, everyone knows that on a hot day it is better not to wear a black T-shirt, as it will make you feel hot. Why? Compared to a white T-shirt, it absorbs more and reflects less light from the environment. Therefore, wearing a white T-shirt will be cooler precisely because it reflects more thermal radiation into the environment. Which car would you rather sit in on a hot day: one upholstered in black or white leather?
Radiation and “black bodies”
Some bodies absorb light falling on them to a greater extent than others. Bodies that absorb all thermal radiation incident on them have a separate name - “black bodies”. A “black body” absorbs 100% of the thermal radiation incident on it, and if it is in equilibrium with its environment, then it emits the same amount of thermal radiation into this environment.
Most physical bodies are located between mirrors and “black bodies”, which, respectively, reflect or absorb all the light falling on them. Ordinary bodies “from the middle to the half”, belonging to this majority, absorb part of the light falling on them and then re-emit it into the environment. Bodies are shiny because they reflect most of the light that hits them. Dark bodies appear this way because they reflect little light falling on them.
You can learn a lot about the physics of black bodies if you start with the question: how much thermal energy does a black body emit at a given temperature? The amount of thermal energy emitted is proportional to the time of emission: for example, in twice the amount of time, twice as much thermal energy is emitted. So you can write the following formula, where \( t \) is the time:
It is not difficult to understand that the amount of thermal radiation is proportional to the total area from which the radiation occurs. Therefore, we can write another formula, where \( A \) is the area from which the radiation occurs:
Somewhere in the formula there must be temperature \( T \): the warmer the body, the more thermal energy it emits. During the experiments, it turned out that the amount of emitted thermal energy is proportional to the temperature to the fourth power, \( T^4 \). Thus it turns out that:
To give the formula a finished form, you need to insert a constant into it, which is found experimentally. The thermal energy emitted by the “black body” is calculated using the Stefan-Boltzmann constant \( \sigma \) included in its formula.
The value of \(\sigma\) is 5.67·10-8 J(s·m2·K4). However, note that this constant is only suitable for “black bodies”, which are ideal emitters. But most bodies are not ideal emitters, so in most cases it is necessary to insert another constant - one that depends on the substance used. This constant is called emissivity (emissivity) - \( e \). Thus, the desired formula, or the Stefan-Boltzmann radiation law, takes the following form:
where \( e \) is the emissivity of the body, \( \sigma \) is the Stefan-Boltzmann constant (5.67 10-8 J(s m2 K4)), \( A \) is the radiating area, \(t\) is time, and \(T\) is the temperature in kelvins.
Let's say that a certain person has an emissivity of approximately 0.8. How much thermal energy does he emit every second, provided that his body temperature is 37°C? First, we need to calculate the area from which thermal radiation occurs. Mathematically taking this person to be a cylinder with a height of 1.6 m and a radius of 0.1 m, you get the total surface area as:
where \( r \) and \( h \) are the radius and height, respectively. To find the total thermal energy emitted by this person, plug the numbers into the Stefan-Boltzmann radiation law formula:
So, it turns out 449 J/sec, or 449 W. This value may seem high since the temperature of the skin is not as high as the temperature of the core of the body, but we are dealing with approximate values here.