What is electromagnetic radiation?
Electromagnetic radiation is vibrations of electric and magnetic fields. The speed of propagation in a vacuum is equal to the speed of light (about 300,000 km/s). In other environments, the speed of radiation propagation is lower.
Electromagnetic radiation is classified according to frequency ranges. The boundaries between the ranges are very arbitrary; there are no sharp transitions in them.
- Visible light. This is the narrowest range in the entire spectrum. A person can only perceive it. Visible light combines the colors of the rainbow: red, orange, yellow, green, blue, indigo, violet. Behind the red color there is infrared radiation, behind the violet there is ultraviolet radiation, but they are no longer distinguishable by the human eye.
Visible light waves are very short and high frequency. The length of such waves is one billionth of a meter or one billion nanometers. Visible light from the Sun is a kind of cocktail in which three primary colors are mixed: red, yellow and blue.
- Ultraviolet radiation is part of the spectrum between visible light and x-rays. Ultraviolet radiation is used to create lighting effects on theater stages and discos; Some countries' banknotes contain security features that are only visible under ultraviolet light.
- Infrared radiation is the part of the spectrum between visible light and short radio waves. Infrared radiation is heat rather than light: every heated solid or liquid emits a continuous infrared spectrum. The higher the heating temperature, the shorter the wavelength and the higher the radiation intensity.
- X-ray radiation (x-ray). X-ray waves have the property of passing through matter without being absorbed too much. Visible light does not have this ability. Thanks to X-rays, some crystals can glow.
- Gamma radiation is the shortest electromagnetic waves that pass through matter without absorption: they can overcome a one-meter concrete wall and a lead barrier several centimeters thick.
IMPORTANT! It is necessary to avoid X-ray and gamma radiation, as they pose a potential danger to humans.
General concept
The properties of electromagnetic oscillations were discovered at the beginning of the 19th century by the English scientist D. C. Maxwell. The physicist believed that electromagnetic waves are perpendicular to the direction of propagation of the wave, its speed. But the electromagnetic field exists separately from the above two. The magnetic and electric fields, interacting with each other, act on the charged particles of the surface of the wave front, creating a field that exists independently and has its own properties.
Electromagnetic waves can propagate in different media, including in a vacuum. The field itself is matter that spreads in the environment. The speed of propagation of an electromagnetic wave in a vacuum is equal to the speed of light, i.e. 3*10 to the 8th power m/s. The meaning does not decay as it passes through space, constantly.
The scale of electromagnetic radiation shows how one qualitative type of radiation transforms into another as the interrelated quantitative indicators of frequency and wavelength change. One type of radiation range is visible light.
Features of electromagnetic radiation
At first glance, it may seem that there is nothing in common between such different phenomena of electromagnetic radiation. And in fact, what do an X-ray tube, a radioactive substance, a warm stove, a flashlight lamp and an alternating current generator connected to a power line have in common, as does the eye, photographic film, thermocouple, radio receiver and television antenna? The second list consists of receivers, and the first list consists of sources of electromagnetic radiation.
The impact of different types of radiation on the human body is also different: X-ray and gamma radiation cause damage to tissues and organs, visible light affects vision, infrared radiation heats the human body, and radio waves are not felt at all. But, despite the obvious differences, all of the above examples of radiation are different sides of the same phenomenon.
All types of electromagnetic waves have the same speed of propagation in free space. However, the number of oscillations per unit time varies widely: for electromagnetic waves in the low-frequency range - from several oscillations per second and up to 1020 oscillations per second in the case of gamma and X-ray radiation.
Since the length of the electromagnetic wave is represented in the form of the expression $l = frac {c}{f}$, it also varies over a wide range - from $10^{-12}$ meters for X-ray radiation and up to several thousand kilometers for low-frequency oscillations. Therefore, the effect of electromagnetic waves on matter is very different in different parts of the spectrum. Electromagnetic waves differ significantly from sound in that they can be transmitted to a source from a receiver through a vacuum.
INFOFIZ
The set of monochromatic components in radiation is called
spectrum
.
Emission spectra
The spectral composition of radiation from substances is very diverse. But despite this, all spectra, as experience shows, can be divided into three types.
Continuous spectra
The continuous spectrum is a continuous multi-colored stripe.
White light has a continuous spectrum.
The solar spectrum or arc light spectrum is continuous. This means that the spectrum contains waves of all wavelengths. There are no breaks in the spectrum, and a continuous multi-colored strip can be seen on the spectrograph screen.
Continuous (or continuous) spectra, as experience shows, are given by bodies in the solid or liquid state, as well as highly compressed gases. To obtain a continuous spectrum, the body must be heated to a high temperature. A continuous spectrum is also produced by high-temperature plasma. Electromagnetic waves are emitted by plasma mainly when electrons collide with ions.
The nature of the continuous spectrum and the very fact of its existence are determined not only by the properties of individual emitting atoms, but also to a strong extent depend on the interaction of atoms with each other.
Radiation from sources in which light is emitted by atoms of matter has a discrete spectrum
. They are divided into:
1. ruled
2. striped
Line spectra
The line spectrum consists of individual colored lines of varying brightness, separated by wide dark bands.
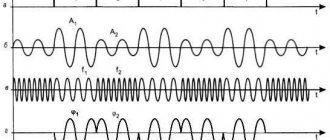
Let's add a piece of asbestos moistened with a solution of ordinary table salt into the pale flame of a gas burner. When observing a flame through a spectroscope, a bright yellow line will flash against the background of the barely visible continuous spectrum of the flame. This yellow line is produced by sodium vapor, which is formed when the molecules of table salt are broken down in a flame. The figure also shows the spectra of hydrogen and helium. Such spectra are called line spectra. The presence of a line spectrum means that a substance emits light only at certain wavelengths (more precisely, in certain very narrow spectral intervals).
Line spectra give all substances in the gaseous atomic (but not molecular) state. In this case, light is emitted by atoms that practically do not interact with each other. This is the most fundamental, basic type of spectra.
Isolated atoms emit strictly defined wavelengths.
Typically, to observe line spectra, the glow of vapor of a substance in a flame or the glow of a gas discharge in a tube filled with the gas under study is used.
As the density of the atomic gas increases, the individual spectral lines expand, and finally, with very high compression of the gas, when the interaction of atoms becomes significant, these lines overlap each other, forming a continuous spectrum.
Striped spectra
The banded spectrum consists of individual bands separated by dark spaces.
With the help of a very good spectral apparatus one can discover that each band is a collection of a large number of very closely spaced lines. Unlike line spectra, striped spectra are created not by atoms, but by molecules that are not bound or weakly bound to each other.
To observe molecular spectra, as well as to observe line spectra, the glow of vapor in a flame or the glow of a gas discharge is usually used.
Absorption spectra
All substances whose atoms are in an excited state emit light waves, the energy of which is distributed in a certain way over wavelengths. The absorption of light by a substance also depends on the wavelength. Thus, red glass transmits waves corresponding to red light and absorbs all others.
If you pass white light through a cold, non-emitting gas, dark lines appear against the background of the continuous spectrum of the source. This will be the absorption spectrum.
The absorption spectrum appears as dark lines against the background of the continuous spectrum of the source.
Gas absorbs most intensely the light of precisely those wavelengths that it emits when highly heated. The dark lines against the background of the continuous spectrum are absorption lines that together form the absorption spectrum.
There are continuous, line and striped absorption spectra.
Various types of electromagnetic radiation, their properties and practical applications.
Electromagnetic wave scale. The boundaries between different ranges are arbitrary
Low frequency vibrations.
Direct current – frequency ν = 0 – 10 Hz
.
Atmospheric interference and alternating current – frequency ν = 10 – 104 Hz
Radio waves.
Frequency ν =104 – 1011 Hz
Wavelength λ = 10-3 – 103 m
Obtained using oscillatory circuits.
Properties.
Radio waves of different frequencies and with different wavelengths are absorbed and reflected differently by media and exhibit diffraction and interference properties.
Application.
Radio communications, television, radar.
Infrared radiation.
Frequency ν =3 1011 – 4 1014 Hz
Wavelength λ = 8·10-7 – 2·10-3 m
Emitted by atoms and molecules of matter.
Infrared radiation is emitted by all bodies at any temperature. A person emits electromagnetic waves λ ≈ 9·10-6 m.
Properties.
- Passes through some opaque bodies, as well as through snow, rain, and haze.
- Produces a chemical effect on photographic plates.
- When absorbed by a substance, it heats it up.
- Causes an internal photoelectric effect in germanium.
- Invisible.
- Capable of interference and diffraction phenomena.
- Recorded by thermal, photoelectric and photographic methods.
Application.
Obtain images of objects in the dark, with night vision devices, and in fog. Used in forensics, physiotherapy. in industry for drying painted products, building walls, wood, fruit.
Visible radiation.
The portion of electromagnetic radiation perceived by the eye (red to violet).
Frequency ν =4·1014 – 8·1014 Hz
Wavelength λ = 8·10-7 – 4·10-7 m
Properties.
It is reflected, refracted, affects the eye, and is capable of the phenomena of dispersion, interference, and diffraction.
Ultraviolet radiation.
Frequency ν =8 1014 – 3 1015 Hz
Wavelength λ = 10-8 – 4 10-7 m
(but less than violet light)
Sources: gas-discharge lamps with quartz tubes (quartz lamps).
Emitted by all solids with temperatures > 1000°C, as well as luminous mercury vapor.
Properties.
- High chemical activity (decomposition of silver chloride, glow of zinc sulfide crystals).
- Invisible.
- Great penetrating power.
- Kills microorganisms.
- In small doses it has a beneficial effect on the human body (tanning), but in large doses it has a negative biological effect: changes in cell development and metabolism, effects on the eyes.
Application.
In medicine, in cosmetology (solarium, tanning), in industry.
X-rays.
Frequency ν =3·1015 – 3·1019 Hz
Wavelength λ = 10-11 – 4 10-8 m
They are emitted when electrons moving with high acceleration are suddenly slowed down.
Obtained using an X-ray tube: electrons in a vacuum tube are accelerated by an electric field at high voltage, reaching the anode, and upon impact are sharply decelerated. When braking, electrons move with acceleration and emit electromagnetic waves with a short length (from 100 to 0.01 nm).
Properties.
- Interference, diffraction of x-rays on a crystal lattice.
- Great penetrating power.
- Irradiation in large doses causes radiation sickness.
Application.
In medicine (diagnosis of diseases of internal organs), in industry (control of the internal structure of various products, welds).
Gamma radiation (γ – radiation).
Frequency ν =3·1020 Hz
and higher
Wavelength λ =3.3·10-11 m
Sources: atomic nucleus (nuclear reactions).
Properties.
- Has enormous penetrating power.
- Has a strong biological effect.
Application.
In medicine, in production (γ - flaw detection).
Electromagnetic radiation scale
The processes occurring in space and the objects that are located there generate electromagnetic radiation. The wave scale is a method of recording electromagnetic radiation.
A detailed illustration of the spectral range is presented in the figure. The boundaries on such a scale are arbitrary.
Reasons for limiting waves to frequency
It would seem that there should exist waves of all frequencies ($\nu $) from $\nu =0\ Hz$ to $\nu =\infty \Hz.$ However, since a light wave has corpuscular properties in addition to wave properties, there are some restrictions . Quantum theory states that electromagnetic radiation is emitted in the form of quanta (portions of energy). The energy of a quantum (W) is related to its frequency by the expression:
where $h=6.62\cdot {10}^{-34}J\cdot s$ is Planck’s constant, $\hbar =\frac{h}{2\pi }=1.05\cdot {10}^{-34}J\cdot s$ - Planck's constant with a bar. From expression (1) it follows that infinite frequencies are impossible, since there are no quanta with infinitely high energy. The same expression imposes restrictions on low frequencies, since there is a minimum value of vant energy ($W_0$), from which it follows that the minimum frequency (${\nu }_0$) is equal to:
Are you an expert in this subject area? We invite you to become the author of the Directory Working Conditions
Note 1
It must be said that to this day the existence of a lower limit on photon energy has not been proven in physics. A minimum frequency of about 8 Hz is observed in standing electromagnetic waves between the ionosphere and the earth's surface.
Main sources of electromagnetic radiation
- Power lines. At a distance of 10 meters, they pose a threat to human health, so they are placed at a high altitude or buried deep in the ground.
- Electric transport. This includes electric cars, electric trains, subways, trams and trolleybuses, as well as elevators. The metro has the most harmful effects. It is better to travel on foot or by your own transport.
- Satellite system. Fortunately, strong radiation, colliding with the surface of the Earth, is dissipated, and only a small part of the danger reaches people.
- Functional transmitters: radars and locators. They emit an electromagnetic field at a distance of 1 km, so all airports and weather stations are located as far as possible from cities.
It will be interesting➡ Arduino for beginners
Radiation from household electrical appliances
Widespread sources of electromagnetic radiation are household appliances that are found in our homes.
- Cell phones. The radiation from our smartphones does not exceed established standards, but when we call someone, after dialing the number, the base station connects to the phone. At this moment, the norm is greatly exceeded, so do not bring the phone to your ear immediately, but a few seconds after dialing the number.
- Computer. The radiation also does not exceed the norm, but during long-term work, SanPin recommends taking a break of 5-15 minutes every hour.
- Microwave. The microwave body provides protection from radiation, but not 100%. Being near a microwave is dangerous: radiation penetrates 2 cm under a person’s skin, triggering pathological processes. When operating the microwave oven, maintain a distance of 1-1.5 meters from it.
- TV. Modern plasma TVs do not pose a great danger, but you should be wary of old ones with picture tubes and keep a distance of at least 1.5 m.
- Hair dryer. When a hair dryer operates, it creates an electromagnetic field of enormous strength. At this time, we dry our hair long enough and hold the hair dryer close to our head. To reduce the danger, use a hair dryer maximum once a week. Drying your hair in the evening can cause insomnia.
- Electric razor. Instead, buy a regular razor, and if you are used to it, buy a battery-powered electric razor. This will significantly reduce the electromagnetic load on the body.
- Chargers create a field in all directions at a distance of 1 m. When charging your gadget, do not be close to it, and after charging, unplug the device from the outlet so that there is no radiation.
- Electrical wiring and sockets. Cables coming from electrical panels pose a particular danger. The distance from the cable to the sleeping place must be at least 5 meters.
- Energy-saving lamps also emit electromagnetic waves. This applies to fluorescent and LED lamps. Install a halogen or incandescent lamp: they do not emit anything and are not dangerous.
Spectrum of electromagnetic radiation
After the appearance of Maxwell's equations, it became clear that they predict the existence of a natural phenomenon unknown to science - transverse electromagnetic waves
, which are oscillations of interconnected electric and magnetic fields propagating in space at the speed of light.
James Clark Maxwell himself was the first to point out to the scientific community this consequence from the system of equations he derived. In this refraction, the speed of propagation of electromagnetic waves in a vacuum turned out to be such an important and fundamental universal constant that it was designated by a separate letter c,
in contrast to all other speeds, which are usually designated by the letter
v.
Having made this discovery, Maxwell immediately determined that visible light was “merely” a type of electromagnetic wave. By that time, the wavelengths of light in the visible part of the spectrum were known - from 400 nm (violet rays) to 800 nm (red rays). (A nanometer is a unit of length equal to one billionth of a meter, which is mainly used in atomic and ray physics; 1 nm = 10–9 m .
) All the colors of the rainbow correspond to different wavelengths that lie within these very narrow limits. However, Maxwell's equations did not contain any restrictions on the possible range of electromagnetic wavelengths. When it became clear that electromagnetic waves of very different lengths must exist, a comparison was actually immediately put forward regarding the fact that the human eye distinguishes such a narrow band of their lengths and frequencies: a person was likened to a listener of a symphony concert, whose hearing is capable of catching only the violin part, not distinguishing all other sounds.
Soon after Maxwell's prediction of the existence of electromagnetic waves in other spectral ranges, a series of discoveries followed that confirmed his correctness. Radio waves were the first to be discovered in 1888 by the German physicist Heinrich Hertz (1857–1894). The only difference between radio waves and light is that the length of radio waves can range from a few decimeters to thousands of kilometers. According to Maxwell's theory, the cause of electromagnetic waves is the accelerated movement of electric charges. Oscillations of electrons under the influence of alternating electrical voltage in the antenna of a radio transmitter create electromagnetic waves that propagate in the earth's atmosphere. All other types of electromagnetic waves also arise as a result of various types of accelerated movement of electrical charges.
Like light waves, radio waves can travel long distances in the Earth's atmosphere with virtually no loss, making them useful carriers of encoded information. Already in early 1894 - just a little over five years after the discovery of radio waves - the Italian engineer-physicist Guglielmo Marconi (1874–1937) designed the first working wireless telegraph - the prototype of modern radio - for which he was awarded the Nobel Prize in 1909 .
After the existence of electromagnetic waves outside the visible spectrum, predicted by Maxwell’s equations, was first experimentally confirmed, the remaining niches of the spectrum were filled very quickly. Today, electromagnetic waves of all ranges without exception have been discovered, and almost all of them find wide and useful applications in science and technology. Frequencies of waves and energies of corresponding quanta of electromagnetic radiation ( see.
Planck's constant) increase with decreasing wavelength.
The totality of all electromagnetic waves forms the so-called continuous spectrum of electromagnetic radiation
.
It is divided into the following ranges
(in order of increasing frequency and decreasing wavelength):
Radio waves
As already noted, radio waves can vary significantly in length - from a few centimeters to hundreds and even thousands of kilometers, which is comparable to the radius of the Earth (about 6400 km). Waves of all radio ranges are widely used in technology - decimeter and ultrashort meter waves are used for television and radio broadcasting in the ultrashort wave range with frequency modulation (VHF/FM), providing high quality signal reception within the zone of direct wave propagation. Radio waves in the meter and kilometer range are used for radio broadcasting and radio communications over long distances using amplitude modulation (AM), which, although at the expense of signal quality, ensures its transmission over arbitrarily large distances within the Earth due to the reflection of waves from the planet’s ionosphere. However, today this type of communication is becoming a thing of the past thanks to the development of satellite communications. Waves in the decimeter range cannot bend around the earth's horizon like meter waves, which limits the reception area to the direct propagation region, which, depending on the height of the antenna and the power of the transmitter, ranges from several to several tens of kilometers. And here satellite repeaters come to the rescue, taking on the role of radio wave reflectors that the ionosphere plays in relation to meter waves.
Microwave
Microwaves and microwave radio waves have a length of 300 mm to 1 mm. Centimeter waves, like decimeter and meter radio waves, are practically not absorbed by the atmosphere and are therefore widely used in satellite and cellular communications and other telecommunication systems. The size of a typical satellite dish is just equal to several wavelengths of such waves.
Shorter microwave waves also have many industrial and domestic applications. It is enough to mention microwave ovens, which are now equipped in both industrial bakeries and home kitchens. The operation of a microwave oven is based on the rapid rotation of electrons in a device called a klystron
.
As a result, electrons emit electromagnetic microwave waves of a certain frequency, at which they are easily absorbed by water molecules. When you put food in a microwave oven, the water molecules in the food absorb the energy from the microwaves, move faster, and thus heat the food. In other words, unlike a conventional oven or oven, where food is heated from the outside, a microwave oven heats it from the inside
.
Infrared rays
This part of the electromagnetic spectrum includes radiation with wavelengths from 1 millimeter to eight thousand atomic diameters (about 800 nm). A person feels the rays of this part of the spectrum directly through the skin - like heat. If you extend your hand towards a fire or a hot object and feel heat emanating from it, you perceive infrared radiation as heat. Some animals (for example, burrow vipers) even have sensory organs that allow them to determine the location of warm-blooded prey by the infrared radiation of its body.
Since most objects on the Earth's surface emit energy in the infrared wavelength range, infrared detectors play an important role in modern detection technologies. Infrared eyepieces of night vision devices allow people to “see in the dark,” and with their help it is possible to detect not only people, but also equipment and structures that have warmed up during the day and give off their heat at night to the environment in the form of infrared rays. Infrared ray detectors are widely used by rescue services, for example, to detect living people under rubble after earthquakes or other natural and man-made disasters.
Visible light
As already mentioned, the electromagnetic wavelengths of the visible light range range from eight to four thousand atomic diameters (800–400 nm). The human eye is an ideal tool for recording and analyzing electromagnetic waves in this range. This is due to two reasons. Firstly, as noted, waves of the visible part of the spectrum propagate almost unhindered in an atmosphere transparent to them. Secondly, the temperature of the solar surface (about 5000°C) is such that the peak energy of solar rays falls precisely in the visible part of the spectrum. Thus, our main source of energy emits a huge amount of energy in the visible light range, and the environment around us is largely transparent to this radiation. It is therefore not surprising that the human eye, in the process of evolution, was formed in such a way as to capture and recognize precisely this part of the spectrum of electromagnetic waves.
I want to emphasize once again that there is nothing special from a physical point of view in the range of visible electromagnetic rays. It is just a narrow strip in a wide spectrum of emitted waves (see figure). For us, it is so important only insofar as the human brain is equipped with a tool for identifying and analyzing electromagnetic waves in this particular part of the spectrum.
Ultra-violet rays
Ultraviolet rays include electromagnetic radiation with a wavelength from several thousand to several atomic diameters (400–10 nm). In this part of the spectrum, radiation begins to affect the functioning of living organisms. Soft
ultraviolet rays in the solar spectrum (with wavelengths approaching the visible part of the spectrum), for example, cause tanning in moderate doses, and severe burns in excess doses.
Hard
(short-wave) ultraviolet radiation is destructive to biological cells and is therefore used, in particular, in medicine to sterilize surgical instruments and medical equipment, killing all microorganisms on their surface.
All life on Earth is protected from the harmful effects of hard ultraviolet radiation by the ozone layer
Earth's atmosphere, absorbing
most
of the hard ultraviolet rays in the spectrum of solar radiation (
see
Ozone hole). If not for this natural shield, life on Earth would hardly have emerged from the waters of the World Ocean. However, despite the protective ozone layer, some of the hard ultraviolet rays reach the Earth's surface and can cause skin cancer, especially in people who are naturally prone to paleness and do not tan well in the sun.
X-rays
Radiation in the wavelength range from several atomic diameters to several hundred diameters of the atomic nucleus is called X-ray. X-rays penetrate the soft tissues of the body and are therefore indispensable in medical diagnostics. As in the case of radio waves, the time gap between their discovery in 1895 and the beginning of practical use, marked by the receipt of the first X-ray in a Paris hospital, was a matter of years. (It is interesting to note that Parisian newspapers at the time were so enamored with the idea that X-rays could penetrate clothing that they reported virtually nothing about their unique medical applications.)
Gamma rays
The shortest wavelength and highest frequency and energy rays in the electromagnetic spectrum are γ rays (gamma rays). They consist of ultra-high energy photons and are used today in oncology to treat cancer tumors (or rather, to kill cancer cells). However, their effect on living cells is so destructive that extreme care must be taken so as not to cause harm to surrounding healthy tissues and organs.
In conclusion, it is important to emphasize once again that although all the described types of electromagnetic radiation manifest themselves externally differently, at their core they are twins. All electromagnetic waves in any part of the spectrum represent transverse oscillations of electric and magnetic fields propagating in a vacuum or medium; they all propagate in a vacuum at the speed of light with
and differ from each other only in wavelength and, as a consequence, in the energy they transfer. It only remains to add that the boundaries of the ranges I have named are quite arbitrary in nature (and in other books you will most likely come across slightly different values of the boundary wavelengths). In particular, microwave emissions with long wavelengths are often and rightly classified as ultra-high-frequency radio waves. There are no clear boundaries between hard ultraviolet and soft X-rays, as well as between hard X-rays and soft gamma radiation.
Types of electromagnetic radiation
EMR is divided into types according to length and frequency characteristics.
The wavelength varies in the following ranges:
Electromagnetic radiation ranges
- Radio waves (from 0.1 mm to 10 km or more) are divided into short, ultra-short, medium, long and ultra-long. Ultrashort radio waves belong to ultra-high frequency (microwave) waves.
- Infrared rays (from 1 mm to 780 nm).
- Ultraviolet rays (from 380 mm to 10 nm).
- Visible light (from 780 mm to 380 nm).
- X-ray radiation (from 10 nm to 5 pm).
- Gamma rays (up to 5 pm).
The frequency of the waves varies from 30 kHz (for radio waves) to 6×10¹9 Hz or more (for gamma rays).
Waves of different lengths are formed in different ways:
- X-rays appear when rapidly moving electrons pass into a state with lower energy due to braking;
- ultraviolet is emitted due to the movement of accelerated electrons;
- infrared radiation is emitted by hot objects;
- radio waves are formed from high-frequency currents moving through antennas;
- Ionizing gamma radiation is emitted during nuclear reactions.
The above types of waves are absorbed differently by substances: X-ray and gamma waves penetrate the tissues of the body and are almost not absorbed, infrared rays pass through a number of opaque objects, and when absorbed, the substance heats up.
Types of electromagnetic waves and their characteristics
| Type of radiation/name of spectrum range | Wavelength, λ | Frequency, f | Photon energy, Eph |
| Low frequency | 100,000 km – 10 km | 3 Hz – 30 kHz | 12.4 feV – 124 peV |
| Radio waves | 10 km – 1 m | 30 kHz – 300 MHz | 124 peV – 1.24 µeV |
| Microwave | 1 m – 1 mm | 300 MHz – 300 GHz | 1.24 µeV – 1.24 meV |
| Infrared radiation/thermal radiation | 1 mm – 780 nm | 300 GHz – 385 THz | 1.24 meV – 1.59 eV |
| Visible light | 780 nm – 380 nm | 385 THz – 789 THz | 1.59 eV – 3.27 eV |
| Ultraviolet radiation | 380 nm – 10 nm | 789 THz – 30 PHz | 3.27 eV – 124 eV |
| X-ray radiation | 10 nm – 10 pm | 30 PHz – 30 EHz | 124 eV – 124 keV |
| Gamma radiation | < 10 pm | > 30 EHz | > 124 keV |
Table: Types of electromagnetic waves and their properties
The more obscure of the unit prefixes used here are “f” for “femto” and 10-15, “p” for “pico” and 10-12, “T” for “tera” and 1012 , "P" for "penta" and 1015, and "E" for "exa" and 1018. Additionally, we have the conversion of 1 eV ≈ 1.602 * 10-19 J through the elementary charge e.
Gamma radiation actually refers to any radiation with a wavelength less than 10 pm. We also see that visible light is only a very small part of the entire electromagnetic spectrum. Finally, it should be noted that this is only a rough classification, and each of these types of radiation is in practice broken down into even more subtypes.
Properties of electromagnetic waves
The spectral characteristics of EM radiation are based on:
- Wavelength is the distance where it remains in one phase.
- Frequency – the number of repetitions per unit of time (second).
- The energy of the photon that carries the waves.
The oscillation frequency is calculated as: λ = сn / υ, where:
- с – speed of electromagnetic waves in vacuum;
- υ – in the environment;
- n – refractive index.
The latter is always greater than one, which means that in any substance an electromagnetic wave propagates more slowly than in a physical vacuum.
Table of properties of electromagnetic waves inherent in radiation of any frequency from the spectrum.
| Peculiarity | Explanation |
| Submission to the law of reflection | The angles of incidence and reflection are equal. The ratio of the sine of the first to the sine of the second is a constant value, it is proportional to the ratio of the propagation speeds in both media. |
| Diffraction | Deviation of waves from a straight path at the edge of obstacles to go around them when passing through holes. |
| Interference | The ability of coherent waves to overlap, as a result of which they are amplified or dampened in certain places. |
| Dispersion | Dependence of the refractive index on the radiation frequency. |
| Absorption | Partially absorbed during the transition between media. |
| Saving frequency | The frequency during the transition of electromagnetic waves between media is maintained. |
| Transverse spread | Electromagnetic radiation is always transverse. |
| Refraction | At the boundary of the media, the main radiation passes further, being refracted, the part is reflected the more strongly, the lower the wave frequency. |
Additional colors of the spectrum
The visible light spectrum contains both primary and secondary colors. How can I get additional colors? Their production is based on the experience of I. Newton, who in 1671, using a prism, decomposed a white beam of sunlight into a spectrum: successively red, orange, yellow, green, blue and violet colors.
Additional colors of the spectrum are obtained in different ways:
Additional colors of the spectrum
- If you divide the spectrum into two parts (red-orange-yellow and green-blue-violet), two mixtures of the first three and the second three will give two colors. The peculiarity of the latter is such that if you put them together with a lens, you get white again.
- If you physically close one color in the spectrum, then collect the remaining colors with a lens, the resulting color will be complementary to the closed one. For example, if you close green, red will gather, closing yellow will create purple. Red will be complementary to green, and purple will be complementary to yellow.
By closing the sequence of colors of the spectrum into a circle, we obtain a diagram called the spectral circle.
Primary additional colors:
- red and green;
- yellow and purple;
- blue and orange.
Table 1. Additional colors.
| Highlighted part | Red | Orange | Yellow | Yellow-green | Green | bluish green |
| Color of the mixture of the remaining rays | Bluish green | Blue | Blue | Violet | Purple | Red |
When mixing additional colors, which has been proven experimentally, it is no longer possible to obtain a pure color - any admixture of an additional color to the main color reduces the saturation.
Scope of application
Somewhere since the end of the 19th century, all human progress has been associated with the practical use of electromagnetic waves.
It will be interesting➡ Switch connection diagram
The first thing worth mentioning is radio communication. It gave people the opportunity to communicate, even if they were far from each other.
Satellite broadcasting and telecommunications are a further development of primitive radio communications.
It is these technologies that have shaped the information image of modern society.
Sources of electromagnetic radiation should be considered both large industrial facilities and various power lines.
Electromagnetic waves are actively used in military affairs (radars, complex electrical devices). Also, medicine could not do without their use. Infrared radiation can be used to treat many diseases.
X-rays help determine damage to a person's internal tissues.
Lasers are used to perform a number of operations that require pinpoint precision.
The importance of electromagnetic radiation in human practical life is difficult to overestimate.
Soviet video about the electromagnetic field:
Electromagnetic wave scale
Electromagnetic waves of different frequencies differ significantly in their properties. Therefore, they can be divided into types by constructing a scale of electromagnetic waves.
Low-frequency (ultra-long) waves ($10^4$ Hz or less)
Electromagnetic waves of this frequency have a long wavelength (on the order of kilometers), they are able to bend around large obstacles, and are able to penetrate into the thickness of water and soil. But they are difficult to generate and accept. In addition, the low frequency determines the low information capacity of such waves. Therefore, although low-frequency electrical vibrations are very widely used in the national economy, electromagnetic waves in this range are used mainly only in scientific research of the Earth.
Radio waves (^4$ Hz – ^{11}$ Hz)
Electromagnetic waves in this range range in length from centimeters to kilometers and are quite easily generated and received. At the same time, radio waves with a frequency of less than 3 MHz bend around the curvature of the Earth quite well, are able to pass through not too thick non-conducting barriers and spread over several hundred kilometers, and radio waves with a frequency of up to 30 MHz are additionally capable of being reflected from the upper layers of the atmosphere and completely bending around the Earth. Therefore, radio waves in these ranges are very widely used for communication.
Radio waves with frequencies above 1 GHz pass very weakly through obstacles and are reflected from them. Therefore, radio waves of this frequency are used in radar.
Light Emission ($10^{11}$ Hz – $10^{18}$ Hz)
Electromagnetic waves in this range have wavelengths from units to thousands of nanometers and include infrared radiation from heated bodies, visible light and ultraviolet radiation. Such waves are generated by heated objects; the higher the temperature, the higher the frequency of the radiation.
Visible light in this range occupies a narrow band $3.5×10^{14}$ Hz – $7.5×10^{14}$ Hz. The transparency of the Earth's atmosphere for this range determines the enormous importance of vision for living beings.
X-ray radiation ($10^{18}$ Hz – $10^{20}$ Hz)
To generate radiation of such frequencies, either very high temperatures or excitation of the atoms of the substance by a stream of particles (this happens in cathode tubes) are necessary, since the wavelength is comparable to the size of the atoms. This radiation has a high penetrating ability through non-conducting substances, which makes it possible to widely use it in medicine and flaw detection.
Gamma radiation ($10^{20}$ Hz and above)
Radiation of such high frequencies is generated by atomic nuclei during nuclear reactions; the wavelength here is comparable to the size of atomic nuclei. Also, gamma radiation is the main component of cosmic rays, in which it has the highest frequencies (and highest energies). Therefore, gamma radiation plays an important role in space research. In addition, since gamma rays have a destructive effect on living tissue, they are used in the treatment of cancer.
Summarizing all that has been said, we can build a table of the scale of electromagnetic waves:
Rice. 3. Electromagnetic wave scale table.
Current methods of protection
The most effective method of protection is considered to be reducing the power of radiating sources or simply leaving the area of its influence. But if at home, thanks to the current SNiP and SanPiN, tension indicators rarely exceed current standards, then in production conditions it is not always possible to avoid such exposure.
Reducing the source power can be achieved in several ways:
- Application of absorbing screens and protective structures.
- Installation of blocking or reflective devices.
Also read: How transformer oil is tested
All such means are classified as collective protection; in addition to them, PPE (personal protective equipment) is also used.
Most EMF protection products are designed for industrial environments. These include:
- Reflective screens, canopies and other structures made of metal mesh, reinforcement, metal sheets. In practice, cheaper structures made of steel, non-ferrous metals and their alloys have been obtained. All these structures must be grounded. The operating principle is based on the appearance of Foucault currents (eddy currents) in the screen materials, which have a similar amplitude but are in antiphase. As a result, the resulting field loses its intensity and cannot pass through the protective structure.
- Absorbing structures are made using polymer materials - polystyrene foam, various types of rubber, foam rubber. Wood impregnated with special compounds also performs well; plates made of ferromagnetic alloys are also used, but this is a more expensive result.
- To impart protective properties to various structures, conductive paints based on powdered graphite, metal oxides, carbon black, and colloidal silver are used. In this case, reflective elements of protection against electromagnetic radiation are obtained.
- Ionizers have also become widespread, making it possible to neutralize static voltage charges arising under the influence of electric and magnetic fields. Such devices are also used in everyday life.
Personal protective equipment includes:
- Workwear and footwear made from fabrics woven with metal threads.
- Safety glasses with metallized coatings that have reflective properties.
- To prevent exposure to infrared radiation, standard insulating suits are used.
- Exposure to ultraviolet radiation is neutralized by protective clothing and goggles or a mask with light filters. A simple example is a set of overalls for an electric welder.
We provided only common solutions that make it possible to neutralize or minimize the effects of electromagnetic radiation. But in everyday conditions such options are of little use.
Practical application of protection methods
Solving home problems related to exposure to electromagnetic fields should begin with a simple check. To do this, it is necessary to determine the level of magnetic and electric field strength in an apartment or house. If the indicators do not go beyond the maximum permissible levels that were discussed, then do not worry, they are calculated with a multiple margin.
If there is a problem, then proven methods are used to reduce the impact of electromagnetic waves:
- Check the presence and connection of sockets to grounding circuits. It is recommended to use these elements with special PE conductor contacts.
- Microwaves and other potentially dangerous household devices are equipped with housings with protective shielding. Operation is not allowed even in a partially disassembled state.
- Stationary equipment must be grounded, for this reason it is important to have sockets with appropriate contacts.
Among other well-known methods of radiation protection, we recommend that possible sources be located at the greatest possible distance. You should not sleep next to a microwave, and it is better to use a mobile phone with a headset. But these are common truths, so we won’t dwell on them.
Let us remind you once again that you should only worry about the effects of electromagnetic radiation if an instrumental test has revealed an increased level of field strength. An apartment saturated with electrical appliances is not a reason to panic; given acceptable standards, there is no threat to health. A tin foil hat can only be used as an extravagant accessory.
Electromagnetic radiation ranges[edit]
Electromagnetic radiation is usually divided into frequency ranges (see table). There are no sharp transitions between the ranges; they sometimes overlap, and the boundaries between them are arbitrary. Since the speed of radiation propagation is constant, the frequency of its oscillations is strictly related to the wavelength in vacuum.
| Range name | Wavelengths, λ | Frequencies, ν | Sources | |
| Radio waves | Extra long | 100 – 10 km | 3 - 30 kHz | Atmospheric phenomena. Alternating currents in conductors and electronic flows (oscillatory circuits). |
| Long | 10 km – 1 km | 30 kHz - 300 kHz | ||
| Average | 1 km – 100 m | 300 kHz - 3 MHz | ||
| Short | 100 m - 10 m | 3 MHz - 30 MHz | ||
| Ultra short | 10 m - 2 mm | 30 MHz - 1.5×1011 Hz | ||
| Optical radiation | Infrared radiation | 1.5×1011 Hz - 6 THz (11 octaves) | Radiation of molecules and atoms under thermal and electrical influences. | |
| Visible radiation | 760 – 400 nm | (1 octave) | ||
| Ultraviolet radiation | 400 - 10 nm | < 3×1016 Hz (5 octaves) | Radiation of atoms under the influence of accelerated electrons. | |
| Hard rays | X-ray radiation | 10 – 5×10−3 nm | 3x1016 - 6x1019 Hz | Atomic processes under the influence of accelerated charged particles. |
| Gamma radiation | < 5×10−3 nm | >6×1019 Hz | Nuclear and space processes, radioactive decay. | |
Radio waves
.
Ultrashort radio waves are usually divided into meter, decimeter waves, centimeter waves, millimeter
and
submillimeter
or
micrometer waves
.
Waves with a length of λ
< 1 m (
ν
> 300 MHz) are also commonly called
microwaves
or
microwaves
.
Hard rays
. The boundaries of the regions of X-ray and gamma radiation can be determined only very conditionally. For general guidance, we can assume that the energy of X-ray quanta lies in the range of 20 eV - 0.1 MeV, and the energy of gamma quanta is more than 0.1 MeV.
Radio waves[edit]
Due to the large values of λ, the propagation of radio waves can be considered without taking into account the atomistic structure of the medium. The only exceptions are the shortest radio waves adjacent to the infrared part of the spectrum. In the radio range, the quantum properties of radiation also have a weak effect.
Radio waves arise when alternating current of the appropriate frequency flows through conductors. And vice versa, an electromagnetic wave passing through space excites a corresponding alternating current in a conductor. This property is used in radio engineering when designing antennas.
The natural source of waves in this range are thunderstorms. It is believed that they are also the source of Schumann's standing electromagnetic waves.
Microwave radiation[edit]
Infrared radiation (Thermal)[edit]
Visible radiation (Optical)[edit]
Visible, infrared and ultraviolet radiation make up the so-called optical region of the spectrum
in the broad sense of the word. The identification of such a region is due not only to the proximity of the corresponding parts of the spectrum, but also to the similarity of the instruments used for its study and developed historically mainly in the study of visible light (lenses and mirrors for focusing radiation, prisms, diffraction gratings, interference devices for studying the spectral composition of radiation and etc.).
The frequencies of waves in the optical region of the spectrum are already comparable to the natural frequencies of atoms and molecules], and their lengths are comparable to molecular sizes and intermolecular distances. Thanks to this, phenomena caused by the atomic structure of matter become significant in this area. For the same reason, along with the wave properties, the quantum properties of light also appear.
The most famous source of optical radiation is the Sun. Its surface (photosphere) is heated to a temperature of 6000 degrees and shines with bright yellow light. It is precisely because we were born near such a star that this part of the spectrum of electromagnetic radiation is directly perceived by our senses].
Radiation in the optical range occurs when bodies are heated (infrared radiation is also called thermal radiation) due to the thermal movement of atoms and molecules. The hotter a body is, the higher the frequency of its radiation. When heated to a certain level, the body begins to glow in the visible range (incandescence), first red, then yellow, and so on. Conversely, radiation from the optical spectrum has a thermal effect on bodies.
In addition to thermal radiation, chemical and biological reactions can serve as a source and receiver of optical radiation. One of the most famous chemical reactions, which is a receiver of optical radiation, is used in photography.
Hard radiation[edit]
In the field of X-ray and gamma radiation, the quantum properties of radiation come to the fore. X-ray radiation occurs when fast charged particles (electrons, protons, etc.) decelerate, as well as as a result of processes occurring inside the electronic shells of atoms. Gamma radiation appears as a result of processes occurring inside atomic nuclei, as well as as a result of the transformation of elementary particles. It also appears when fast charged particles decelerate.
Luminous flux unit
1 lumen is the light emitted by a source with a luminous intensity of 1 candela within a solid angle of 1 steradian. A 100-watt incandescent lamp produces approximately 1,000 lumens of light. The brighter the light source, the more lumens it emits.
It will be interesting➡ What is active power?
In addition to lumens, there are other units of measurement that allow us to characterize light. It is possible to measure the spatial and surface flux density - this is how we know the luminous intensity and illumination. Luminous intensity is measured in candelas, illuminance in lux. But it is more important for the consumer to understand in what units the brightness of light bulbs and other lighting devices is indicated when selling. Some manufacturers report the number of lumens divided by watt. This is how luminous efficiency (luminous output) is measured: how much light a lamp produces using 1 watt.
Defining formulas
Since any light source emits it unevenly, the number of lumens does not fully describe the lighting fixture. You can calculate the intensity of light in candelas by dividing its flux, expressed in lumens, by the solid angle, measured in steradians. Using this formula, it will be possible to take into account the totality of rays coming from the source when they intersect the surface of an imaginary sphere, forming a circle on it.
But the question arises what the number of candelas we find gives in practice; It is impossible to find a suitable LED or flashlight based only on the luminous intensity parameter; you also need to take into account the ratio of the scattering angle, which depends on the design of the device. When choosing lamps that shine evenly in all directions, it is important to understand whether they are suitable for the buyer's purposes.
If previously light bulbs for different rooms were selected based on the number of watts, then before purchasing LED lamps you will have to calculate their total brightness in lumens, and then divide this figure by the area of the room. This is how illumination is calculated, which is measured in lux: 1 lux is 1 lumen per 1 m². There are lighting standards for rooms for various purposes.
What is luminous flux?
Light flux is the power of light radiation visible to the human eye; light energy emitted by a surface (luminous or reflective). Luminous flux energy is measured in lumen-seconds and corresponds to 1 lumen emitted or perceived in 1 second. This indicator describes the total flow, without taking into account the concentrating efficiency of the entire device. This assessment also includes scattered, useless light, so the same number of lumens may appear in sources of different designs.
It is necessary to distinguish between the luminous value and the energy value - the latter characterizes light regardless of its property of causing visual sensations. Each photometric light quantity has an analogue that can be expressed quantitatively in units of energy or power. For light energy, this analogue is radiant energy, measured in joules.
Exposure to EM fields can cause:
- dizziness
- headache
- insomnia
- fatigue
- deterioration in concentration
- depressive state
- increased excitability
- irritability
- sudden mood swings
- strong surges in blood pressure
- weakness
- heart muscle dysfunction
- deterioration of myocardial conductivity
- arrhythmia
The danger also lies in the fact that having noticed any of the signs described above, a person will begin to suspect anything but electromagnetic fields caused, for example, by hidden wiring running along the bed.
Infrared, light and ultraviolet radiation
Infrared, light, ultraviolet
radiation constitutes
the optical region of the spectrum of electromagnetic waves
in the broad sense of the word.
This spectrum occupies the range of electromagnetic wavelengths in the range from 2·10-6m = 2μm to 10-8m = 10nm (frequency from 1.5·1014Hz to 3·1016Hz). The upper limit of the optical range is determined by the long-wave limit of the infrared range, and the lower limit by the short-wave limit of the ultraviolet (Fig. 2.14). The proximity of the spectral regions of the listed waves determined the similarity of the methods and instruments used for their research and practical application.
Historically, lenses, diffraction gratings, prisms, diaphragms, and optically active substances included in various optical devices (interferometers, polarizers, modulators, etc.) were used for these purposes. On the other hand, radiation from the optical region of the spectrum has general patterns of transmission of various media, which can be obtained using geometric optics, widely used for calculations and construction of both optical devices and optical signal propagation channels. Infrared radiation is visible to many arthropods (insects, spiders, etc.) and reptiles (snakes, lizards, etc.)
, accessible to semiconductor sensors (infrared photoarrays), but it is not transmitted by the thickness of the Earth’s atmosphere, which does
not allow
infrared stars - “brown dwarfs”, which make up more than 90% of all stars in the Galaxy, to be observed from the Earth’s surface.
The frequency width of the optical range is approximately 18 octaves, of which the optical range accounts for approximately one octave (); for ultraviolet - 5 octaves (), for infrared radiation - 11 octaves (
).
In the optical part of the spectrum, phenomena caused by the atomic structure of matter become significant. For this reason, along with the wave properties of optical radiation, quantum properties appear.
Light
| Light, light, visible radiation - the part of the optical spectrum of electromagnetic radiation visible to the eyes of humans and primates, occupies the range of electromagnetic wavelengths in the range from 400 nanometers to 780 nanometers, that is, less than one octave - a twofold change in frequency. Rice. 1.14. Electromagnetic wave scale Verbal memory meme of the order of colors in the light spectrum: “ K Every |
Safety rules for exposure to electromagnetic radiation on the human body
The best protection against EM radiation is distance.
The radiation density decreases significantly with distance. Each source has a fairly limited radius of action of the fields, so proper planning of places for rest/leisure, work and sleep is already the key to your health, however, you should not forget that any de-energized source of EM fields ceases to be such.
Therefore, do not forget to turn off unused devices from the network, do not place powerful EMR sources near your head, monitor the condition of household appliances and read the instructions for the proper use of household appliances.
The more expensive electronics are, the safer they are?
In theory, high-quality household appliances will be more harmless, since the larger and more “renowned” the manufacturer, the more he will care about his image and, accordingly, certify all his products as responsibly as possible. But this, of course, also affects the cost of the equipment.
However, it is worth considering that this only applies to new equipment that has not been subjected to physical impact, repairs, with proper operation, location, etc. If at least something has been disturbed, then the intensity of the radiation can change significantly.
Previous
MiscellaneousVVG cable: characteristics, marking, device, application