Each measurement is a comparison of the measured quantity with another homogeneous quantity, which is considered unitary. Theoretically, the units for all quantities in physics can be chosen to be independent of each other. But this is extremely inconvenient, since for each value one should enter its own standard. In addition, in all physical equations that reflect the relationship between different quantities, numerical coefficients would arise.
The main feature of the currently used systems of units is that there are certain relationships between units of different quantities. These relationships are established by the physical laws (definitions) that relate the measured quantities to each other. Thus, the unit of speed is chosen in such a way that it is expressed in terms of units of distance and time. When selecting speed units, the speed definition is used. The unit of force, for example, is established using Newton's second law.
When constructing a specific system of units, several physical quantities are selected, the units of which are set independently of each other. Units of such quantities are called basic. The units of other quantities are expressed in terms of the basic ones, they are called derivatives.
Table of units of measurement “Space and time”
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Length | l, s, d | meter | m | The extent of an object in one dimension. | |
| Square | S | square meter | m2 | The extent of an object in two dimensions. | |
| Volume, capacity | V | cubic meter | m3 | The extent of an object in three dimensions. | extensive quantity |
| Time | t | second | With | Duration of the event. | |
| Flat angle | α , φ | radian | glad | The amount of change in direction. | |
| Solid angle | α , β , γ | steradian | Wed | Part of space | |
| Linear speed | v | meter per second | m/s | The speed of changing body coordinates. | vector |
| Linear acceleration | a,w | meters per second squared | m/s2 | The rate of change in the speed of an object. | vector |
| Angular velocity | ω | radians per second | rad/s = (s−1) | Angle change rate. | |
| Angular acceleration | ε | radian per second squared | rad/s2 = (s−2) | Rate of change of angular velocity |
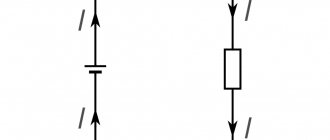
Series and parallel connection of capacitors
Capacitors can be connected in series or in parallel, resulting in a set with new characteristics.
Parallel connection
If you connect capacitors in parallel, then the total capacity of the resulting battery is equal to the sum of all the capacitances of its components. If the battery consists of capacitors of identical design, this can be considered as adding the area of all the plates. In this case, the voltage on each battery cell will be the same, and the charges will add up. For three parallel connected capacitors:
- U=U1=U2=U3;
- q=q1+q2+q3;
- C=C1+C2+C3.
Table of units of measurement "Mechanics"
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Weight | m | kilogram | kg | A quantity that determines the inertial and gravitational properties of bodies. | extensive quantity |
| Density | ρ | kilogram per cubic meter | kg/m3 | Mass per unit volume. | intensive quantity |
| Surface density | ρA | Mass per unit area. | kg/m2 | Ratio of body mass to surface area | |
| Linear density | ρl | Mass per unit length. | kg/m | Ratio of body mass to its linear parameter | |
| Specific volume | v | cubic meter per kilogram | m3/kg | Volume occupied by a unit mass of a substance | |
| Mass flow | Qm | kilogram per second | kg/s | The mass of a substance that passes through a given cross-sectional area of a flow per unit time | |
| Volume flow | Qv | cubic meter per second | m3/s | Volume flow of liquid or gas | |
| Pulse | P | kilogram-meter per second | kg•m/s | Product of mass and speed of a body. | extensive, conserved quantity |
| Momentum | L | kilogram-meter squared per second | kg•m2/s | A measure of the rotation of an object. | conserved quantity |
| Moment of inertia | J | kilogram meter squared | kg•m2 | A measure of the inertia of an object during rotation. | tensor quantity |
| Strength, weight | F, Q | newton | N | An external cause of acceleration acting on an object. | vector |
| Moment of power | M | newton meter | N•m = (kg m2/s2) | The product of a force and the length of a perpendicular drawn from a point to the line of action of the force. | vector |
| Impulse force | I | newton second | N•s | Product of force and the duration of its action | vector |
| Pressure, mechanical stress | p , σ | pascal | Pa = ( kg/(m s2)) | Force per unit area. | intensive quantity |
| Job | A | joule | J = (kg m2/s2) | Dot product of force and displacement. | scalar |
| Energy | E, U | joule | J = (kg m2/s2) | The ability of a body or system to do work. | extensive, conserved quantity, scalar |
| Power | N | watt | W = (kg m2/s3) | Rate of change of energy. |
Law of gravitation
Every object in the Universe is attracted to every other object with a force proportional to their masses and inversely proportional to the square of the distance between them.
${\large F = G \cdot \dfrac {m \cdot M}{R^2}}$
We can add that any body reacts to a force applied to it with acceleration in the direction of this force, in magnitude inversely proportional to the mass of the body.
${\large G}$ — gravitational constant
${\large M}$ — mass of the earth
${\large R}$ — radius of the earth
${\large G = 6.67 \cdot {10^{-11}} \left ( \dfrac {m^3}{kg \cdot {sec}^2} \right ) }$
${\large M = 5.97 \cdot {10^{24}} \left ( kg \right ) }$
${\large R = 6.37 \cdot {10^{6}} \left ( m \right ) }$
Within the framework of classical mechanics, gravitational interaction is described by Newton's law of universal gravitation, according to which the force of gravitational attraction between two bodies of mass ${\large m_1}$ and ${\large m_2}$ separated by a distance ${\large R}$ is
${\large F = -G \cdot \dfrac {m_1 \cdot m_2}{R^2}}$ Here ${\large G}$ is the gravitational constant equal to ${\large 6.673 \cdot {10^{- 11}} m^3 / \left ( kg \cdot {sec}^2 \right ) }$. The minus sign means that the force acting on the test body is always directed along the radius vector from the test body to the source of the gravitational field, i.e. gravitational interaction always leads to the attraction of bodies. The gravity field is potential. This means that you can introduce the potential energy of gravitational attraction of a pair of bodies, and this energy will not change after moving the bodies along a closed loop. The potentiality of the gravitational field entails the law of conservation of the sum of kinetic and potential energy, which, when studying the motion of bodies in a gravitational field, often significantly simplifies the solution. Within the framework of Newtonian mechanics, gravitational interaction is long-range. This means that no matter how a massive body moves, at any point in space the gravitational potential and force depend only on the position of the body at a given moment in time.
Table of units of measurement “Periodic phenomena, oscillations and waves”
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Period | T | second | With | The period of time during which the system makes one complete oscillation | |
| Batch frequency | v, f | hertz | Hz = (s−1) | The number of repetitions of an event per unit of time. | |
| Cyclic (circular) frequency | ω | radians per second | rad/s | Cyclic frequency of electromagnetic oscillations in an oscillatory circuit. | |
| Rotation frequency | n | second to the minus first power | s-1 | A periodic process equal to the number of complete cycles completed per unit of time. | |
| Wavelength | λ | meter | m | The distance between two points in space closest to each other at which the oscillations occur in the same phase. | |
| Wave number | k | meter to the minus first power | m-1 | Spatial wave frequency |
Newton's second and third laws
The interaction of bodies can be described using the concept of force. Force is a vector quantity that is a measure of the influence of one body on another. Being a vector, force is characterized by its modulus (absolute value) and direction in space. In addition, the point of application of the force is important: the same force in magnitude and direction, applied at different points of the body, can have different effects. So, if you grab the rim of a bicycle wheel and pull tangentially to the rim, the wheel will begin to rotate. If you pull along the radius, there will be no rotation.
Newton's second law
The product of the body mass and the acceleration vector is the resultant of all forces applied to the body:
${\large m \cdot \overrightarrow{a} = \overrightarrow{F} }$
Newton's second law relates acceleration and force vectors. This means that the following statements are true.
- ${\large m \cdot a = F}$, where ${\large a}$ is the acceleration modulus, ${\large F}$ is the resulting force modulus.
- The acceleration vector has the same direction as the resultant force vector, since the mass of the body is positive.
Newton's third law
Two bodies act on each other with forces equal in magnitude and opposite in direction. These forces have the same physical nature and are directed along a straight line connecting their points of application.
Table of units of measurement “Thermal phenomena”
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Temperature | T | kelvin | TO | The average kinetic energy of the object's particles. | Intensive value |
| Temperature coefficient | α | kelvin to the minus first power | K-1 | Dependence of electrical resistance on temperature | |
| Temperature gradient | gradT | kelvin per meter | K/m | Change in temperature per unit length in the direction of heat propagation. | |
| Heat (amount of heat) | Q | joule | J = (kg m2/s2) | Energy transferred from one body to another by non-mechanical means | |
| Specific heat | q | joule per kilogram | J/kg | The amount of heat that must be supplied to a substance taken at its melting point in order to melt it. | |
| Heat capacity | C | joule per kelvin | J/C | The amount of heat absorbed (released) by a body during the heating process. | |
| Specific heat | c | joule per kilogram kelvin | J/(kg•K) | Heat capacity of a unit mass of a substance. | |
| Entropy | S | joule per kilogram | J/kg | A measure of the irreversible dissipation of energy or the uselessness of energy. |
What is n equal to in physics if it is the refractive index?
Typically, tables give values for the absolute refractive indices of various substances. Do not forget that this value depends not only on the properties of the medium, but also on the wavelength. Table values of the refractive index are given for the optical range.
| Wednesday | Absolute refractive index |
| air | 1,00029 |
| ice | 1,31 |
| water | 1,33298 |
| ethanol | 1,36 |
| sugar | 1,56 |
| diamond | 2,419 |
So, it became clear what n is in physics. To avoid any questions, it is worth considering some examples.
Table of units of measurement "Molecular physics"
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Quantity of substance | v, n | mole | mole | The number of similar structural units that make up a substance. | Extensive value |
| Molar mass | M , μ | kilogram per mole | kg/mol | The ratio of the mass of a substance to the number of moles of that substance. | |
| Molar energy | Hmol | joule per mole | J/mol | Energy of a thermodynamic system. | |
| Molar heat capacity | resins | joule per mole kelvin | J/(mol•K) | The heat capacity of one mole of a substance. | |
| Molecular concentration | c, n | meter to the minus third power | m-3 | The number of molecules contained in a unit volume. | |
| Mass concentration | ρ | kilogram per cubic meter | kg/m3 | The ratio of the mass of a component contained in a mixture to the volume of the mixture. | |
| Molar concentration | resins | mole per cubic meter | mol/m3 | The content of the component relative to the entire mixture. | |
| Ion mobility | V , μ | square meter per volt second | m2/(V•s) | The proportionality coefficient between the drift velocity of carriers and the applied external electric field. |
What physical quantity can be denoted by n and N?
Its name comes from the Latin word numerus, translated as “number”, “quantity”. Therefore, the answer to the question of what n means in physics is quite simple. This is the number of any objects, bodies, particles - everything that is discussed in a certain task.
Moreover, “quantity” is one of the few physical quantities that do not have a unit of measurement. It's just a number, without a name. For example, if the problem involves 10 particles, then n will simply be equal to 10. But if it turns out that the lowercase “en” is already taken, then you have to use a capital letter.
Table of units of measurement "Electricity and magnetism"
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Current strength | I | ampere | A | Charge flowing per unit time. | |
| Current Density | j | ampere per square meter | A/m2 | The strength of the electric current flowing through a surface element of unit area. | Vector quantity |
| Electric charge | Q, q | pendant | Cl = (A s) | The ability of bodies to be a source of electromagnetic fields and to take part in electromagnetic interaction. | extensive, conserved quantity |
| Electric dipole moment | p | coulomb meter | Kl•m | Electrical properties of a system of charged particles in the sense of the field it creates and the effect of external fields on it. | |
| Polarization | P | pendant per square meter | C/m2 | Processes and states associated with the separation of any objects, mainly in space. | |
| Voltage | U | volt | IN | Change in potential energy per unit charge. | scalar |
| Potential, EMF | φ, σ | volt | IN | The work of external forces (non-Coulomb) to move a charge. | |
| Electric field strength | E | volt per meter | V/m | The ratio of the force F acting on a stationary point charge placed at a given point in the field to the magnitude of this charge q | |
| Electrical capacity | C | farad | F | A measure of a conductor's ability to store electrical charge | |
| Electrical resistance | R,r | ohm | Ohm = (m2 kg/(s3 A2)) | resistance of an object to the passage of electric current | |
| Electrical resistivity | ρ | ohm meter | Ohm•m | The ability of a material to prevent the passage of electric current | |
| Electrical conductivity | G | Siemens | Cm | The ability of a body (medium) to conduct electric current | |
| Magnetic induction | B | tesla | Tl | Vector quantity, which is the force characteristic of the magnetic field | Vector quantity |
| Magnetic flux | F | weber | Wb = (kg/(s2 A)) | A value that takes into account the intensity of the magnetic field and the area it occupies. | |
| Magnetic field strength | H | ampere per meter | Vehicle | The difference between the magnetic induction vector B and the magnetization vector M | Vector quantity |
| Magnetic moment | pm | ampere square meter | A•m2 | A quantity characterizing the magnetic properties of a substance | |
| Magnetization | J | ampere per meter | Vehicle | A quantity characterizing the magnetic state of a macroscopic physical body. | vector quantity |
| Inductance | L | Henry | Gn | The proportionality coefficient between the electric current flowing in any closed circuit and the total magnetic flux | |
| Electromagnetic energy | N | joule | J = (kg m2/s2) | Energy contained in an electromagnetic field | |
| Volumetric energy density | w | joule per cubic meter | J/m3 | Electric field energy of a capacitor | |
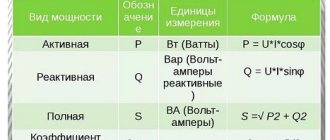
| Active power | P | watt | W | AC power | |
| Reactive power | Q | var | var | A quantity characterizing the loads created in electrical devices by fluctuations in the energy of the electromagnetic field in the alternating current circuit | |
| Full power | S | watt-ampere | W•A | Total power, taking into account its active and reactive components, as well as deviations of the current and voltage waveforms from harmonic |
Table 3 IMPORTANT SI DERIVATIVE UNITS
| Magnitude | Unit | |||||||
| Name | Dimension | Name | Designation | |||||
| international | Russian | |||||||
| Space and time | ||||||||
| Square | L2 | square meter | m2 | m2 | ||||
| Volume, capacity | L3 | cubic meter | m3 | m3 | ||||
| Speed | LT-1 | meter per second | m/s | m/s | ||||
| Acceleration | LT-2 | meters per second squared | m/s2 | m/s2 | ||||
| Angular velocity | T+1 | radians per second | rad/s | rad/s | ||||
| Angular acceleration | T-2 | radian per second squared | rad/s2 | rad/s2 | ||||
| Periodic phenomena, oscillations and waves | ||||||||
| Period | T | second | s | With | ||||
| Periodic process frequency, oscillation frequency | T-1 | hertz | Hz | Hz | ||||
| Rotation frequency | T-1 | second to the minus first power | s-1 | s-1 | ||||
| Wavelength | L | meter | m | m | ||||
| Wave number | L-1 | meter to the minus first power | m-1 | m-1 | ||||
| Attenuation coefficient | T-1 | second to the minus first power | s-1 | s-1 | ||||
| Attenuation coefficient, phase coefficient, propagation coefficient | L-1 | meter to the minus first power | m-1 | m-1 | ||||
| Mechanics | ||||||||
| Density | L-3M | kilogram per cubic meter | kg/m3 | kg/m | ||||
| Specific volume | L3M-1 | cubic meter per kilogram | m3×kg× | m3×kg | ||||
| Quantity of movement | LMT-1 | kilogram-meter per second | kg×m/s | kg×m/s | ||||
| Momentum | L2MT-1 | kilogram meter squared per second | kg×m2/s | kg×m2/s | ||||
| Moment of inertia (dynamic moment of inertia) | L2M | kilogram meter squared | kg×m2 | kg×m2 | ||||
| Force, gravity (weight) | LMT-1 | newton | N | N | ||||
| Moment of force, moment of a couple of forces | L2MT-2 | newton meter | N×m | N×m | ||||
| Impulse force | LMT-1 | newton second | N×s | N×s | ||||
| Pressure, normal stress, shear stress, modulus longitudinal elasticity, shear modulus, bulk modulus | L-1MT-2 | pascal | Pa | Pa | ||||
| Moment of inertia (second moment) of the area of a flat figure - (axial, polar, centrifugal) | L4 | meter to the fourth power | m4 | m4 | ||||
| Moment of resistance of a plane figure | L3 | meter to the third power | m3 | m3 | ||||
| Dynamic viscosity | L-1MT-1 | pascal second | Pa×s | Pa×s | ||||
| Kinematic viscosity | L2T-1 | square meter per second | nr/s | m2/s | ||||
| Surface tension | MT-2 | newton per meter | N/m | N/m | ||||
| Work, energy Power | L2MT-3 L2MT-3 | joule watt | JW | J W | ||||
| Heat | ||||||||
| Celsius temperature | Ө | degrees Celsius | °C | °C | ||||
| Temperature coefficient | Ө-1 | kelvin to the minus first power | K-1 | K-1 | ||||
| Temperature gradient | L-1 Ө | kelvin per meter | K/m | K/m | ||||
| Heat, amount of heat | L2MT-2 | joule | J | J | ||||
| Heat flow | L2MT-3 | watt | W | W | ||||
| Surface heat flux density | MT3 | watt per square meter | W/m2 | W/m2 | ||||
| Thermal conductivity | LMT-3 | watt per meter kelvin | W/(m×K) | W/(m×K) | ||||
| Heat transfer coefficient, heat transfer coefficient | MT-1 Ө-1 | watt per square meter kelvin | W/(m2×K) | W/(m×K) | ||||
| Thermal diffusivity | L2T-1 | square meter per second | m2/s | m2/s | ||||
| Heat capacity | L2MT-2Ө-1 | joule per kelvin | J/K | J/C | ||||
| Specific heat | LT-1Ө-1 | joule per kilogram kelvin | J/(kg×K) | J/(kg×K) | ||||
| Entropy | LMT-1Ө-1 | joule per kelvin | J/K | J/C | ||||
| Specific entropy | L2T-2Ө-1 | joule per kilogram kelvin | J/(kg×K) | J/kg×K) | ||||
| Thermodynamic potential (internal energy, enthalpy, isochoric-isothermal potential, isobaric-isothermal potential), heat of phase transformation, heat of chemical reaction | L1MT-2 | joule | J | J | ||||
| Specific amount of heat, specific thermodynamic potential, specific heat of phase transformation, specific heat of chemical reaction | L2T-2 | joule per kilogram | J/kg | J/kg | ||||
| Electricity and magnetism | ||||||||
| Amount of electricity (electric charge) | T.I. | pendant | WITH | Cl | ||||
| Spatial density of electric charge | L-3-TI | pendant per cubic meter | C/m3 | C/m3 | ||||
| Surface electric charge density | L-2TI | pendant per square meter | C/m2 | C/m2 | ||||
| Electric field strength | LMT-3I-1 | volt per meter | V/m | V/m | ||||
| Electrical voltage | L2MT-3I-1 | volt | V | IN | ||||
| Electric potential | L2MT-3I-1 | volt | V | IN | ||||
| Electric potential difference | L2MT-3I-1 | volt | V | IN | ||||
| Electromotive force | L2M T-3I-1 | volt | V | IN | ||||
| Electrical displacement flux | T.I. | pendant | WITH | Cl | ||||
| Electrical bias | L-2TI | pendant per square meter | C/m2 | C/m2 | ||||
| Electrical capacity | L-2M-1T4I2 | farad | F | F | ||||
| Absolute dielectric constant | L-3M-1T4I2 | farad per meter | F/m | F/m | ||||
| Electric dipole moment | LTI | coulomb meter | C×m | Kl×m | ||||
| Electric current density | L-2I | ampere per square meter | A/m2 | A/m2 | ||||
| Linear electric current density | L-1I | ampere per meter | A/m | Vehicle | ||||
| Magnetic field strength | L-1I | ampere per meter | A/m | Vehicle | ||||
| Magnetomotive force, magnetic potential difference | I | ampere | A | A | ||||
| Magnetic induction | M T-1I-1 | tesla | T | Tl | ||||
| Magnetic flux | L2M T-2I-1 | weber | Wb | Wb | ||||
| Inductance, mutual inductance | L2MT2I2 | Henry | N | Gn | ||||
| Absolute magnetic permeability | LMT-2I-2 | henry per meter | N/t | Gn/m | ||||
| Magnetic moment (amperian) | L2I | ampere square meter | А×m2 | A×m2 | ||||
| Magnetic moment (Coulomb) | L3MT-2I-2 | weber meter | Wb×m | Wb×m | ||||
| Magnetization (magnetization intensity) | L-1I | ampere per meter | A/t | Vehicle | ||||
| Electrical resistance (active, reactive, total) | L2МT-3I-2 | ohm | Ω | Ohm | ||||
| Electrical conductivity (active, reactive, total) | L-2M-1T3I-2 | Siemens | S | Cm | ||||
| Electrical resistivity | L3MT-3I-2 | ohm meter | Ω×m | Ohm×m | ||||
| Electrical conductivity | L-3M-1T3I-2 | siemens per meter | S/m | Sm/m | ||||
| Reluctance | L-2M-1T2I2 | Henry to the minus one | N-1 | Gn-1 | ||||
| Magnetic conductivity | L2МT-2I-2 | Henry | N | Gn | ||||
| Active power | L2MT-3 | watt | W | W | ||||
| Electromagnetic energy | L2MT-2 | joule | J | J | ||||
| Light and other electromagnetic radiation | ||||||||
| Radiation energy | L2MT-2 | joule | J | J | ||||
| Energy exposure (radiant exposure) | MT-2 | joule per square meter | J/m2 | J/m2 | ||||
| Radiation flux, radiation power | L2 MT-3 | watt | W | W | ||||
| Surface radiation flux density, energy luminosity (emissivity), energy illumination (irradiance) | MT-3 | watt per square meter | W/m2 | W/m2 | ||||
| Energy luminous intensity (radiant intensity) | L2 MT-3 | watt per steradian | W/sr | Tue/Wed | ||||
| Energy brightness (radiance) | MT-3 | watt per steradian square meter | W/fsr×m2) | W/(sr×m2) | ||||
| Light flow | J | lumen | lm | lm | ||||
| Light energy | T.J. | lumen-second | lm×s | lm×s | ||||
| Brightness | L-2J | candela per square meter | cd/m2 | cd/m | ||||
| Luminosity | L-2J | lumen per square meter | lm/m2 | lm/m | ||||
| Illumination | L-2J | luxury | Ix | OK | ||||
| Light exposure | L-2TJ | lux-second | lx×s | lx/s | ||||
| Acoustics | ||||||||
| Period of sound vibrations | T | second | s | With | ||||
| Sound frequency | T-1 | hertz | Hz | Hz | ||||
| Sound pressure, sound pressure | L-1MT-2 | pascal | Pa | Pa | ||||
| Oscillatory velocity (the speed at which a particle oscillates) | LT-1 | meter per second | m/s | m/s | ||||
| Volume velocity | L3T-1 | cubic meter per second | m3/s | m3/s | ||||
| Sound speed | LT-1 | meter per second | m/s | m/s | ||||
| Sound energy | L2MT-2 | joule | J | J | ||||
| Sound Energy Density | L-1MT-2 | joule per cubic meter | J/m3 | J/m3 | ||||
| Flow of sound energy | L2MT-3 | watt | W | W | ||||
| Sound power | L2MT-3 | watt | W | W | ||||
| Sound intensity | MT-3 | watt per square meter | W/m2 | W/m2 | ||||
| Acoustic impedance | L4MT-1 | pascal second per cubic meter | Pa×s/m3 | Pa×s/m3 | ||||
| Specific acoustic resistance | L-2MT-1 | pascal second per meter | Pa×s/m | Pa×s/m | ||||
| Mechanical resistance | MT-1 | newton second per meter | N×s/m | N×s/m | ||||
| Equivalent absorption area of a surface or object | L2 | square meter | m2 | m2 | ||||
| Reverberation time | T | second | s | With | ||||
| Physical chemistry and molecular physics | ||||||||
| Molar mass | MN-1 | kilogram per mole | kg/mol | kg/mol | ||||
| Molar volume | L3N-1 | cubic meter per mole | m3/mol | m3/mol | ||||
| Thermal effect of a chemical reaction (formation, dissolution, combustion, phase transformations and etc.) | L2MT-2 | joule | J | J | ||||
| Molar internal energy, molar enthalpy, chemical potential, chemical affinity, activation energy | L2MT2N-1 | joule per mole | J/mol | J/mol | ||||
| Molar heat capacity, molar entropy | L2MT-2 Ө-1N-1 | joule per mole kelvin | J/(mol×K) | J/(mol×K) | ||||
| Molecular concentration | L-3 | meter to the minus third power | m-3 | m-3 | ||||
| Mass concentration | M L-3 | kilogram per cubic meter | kg/m3 | kg/m3 | ||||
| Molar concentration | L-3N | mole per cubic meter | mol/m3 | mol/m3 | ||||
| Molality. specific adsorption | M-3N | mole per kilogram | mol/kg | mol/kg | ||||
| Volatility (fugacity) | L-1mt2 | pascal | Pa | Pa | ||||
| Osmotic pressure | L-1ML-2 | pascal | Pa | Pa | ||||
| Diffusion coefficient | L2T-1 | square meter per second | m2/s | m2/s | ||||
| Chemical reaction rate | L-3 T-1N | moles per cubic meter per second | mol/(m3×s) | mol/(m3×s) | ||||
| Degree of dispersion | L-1 | meter to the minus first power | m-1 | m-1 | ||||
| Specific surface area | L2M-1 | square meter per kilogram | m2/kg | m2/kg | ||||
| Surface density | L-2N | moles per square meter | mol/m2 | mol/m2 | ||||
| Electric dipole moment | LTI | coulomb meter | C×m | Kl×m | ||||
| Polarization | М-1Т4I2 | coulomb square meter per volt | C×m2/V | Kl×m2/V | ||||
| Molecular refraction | М-1Т4I2N-1 | coulomb-square meter per volt-mole | C×m2/(V×mol) | Kl×m2/ (V×mol) | ||||
| Ionic strength of solution | M-1 N M-1 T3 I2 N-1 | mole per kilogram Siemens square meter per mole | mol/kg S×m2/mol | mol/kg cm×m2/mol | ||||
| Electrode potential | L2MT-3 I-1 | volt | V | IN | ||||
| Molar concentration | L-3N | mole per cubic meter | mol/m3 | mol/m3 | ||||
| Ion mobility | M-1T2I | square meter per volt second | m2/(V×s) | m2/(W×s) | ||||
| Ionizing radiation | ||||||||
| Energy of ionizing radiation | L2MT-2 | joule | J | J | ||||
| Absorbed radiation dose (radiation dose), kerma | L2 T 2 | Gray | Gy | Gr | ||||
| Exposure dose X-ray and gamma radiation | M-1TI | pendant per kilogram | C/kg | C/kg | ||||
| Activity of a nuclide in a radioactive source | T-1 | bskkerel | Bq | Bk | ||||
| Atomic and nuclear physics | ||||||||
| Rest mass of a particle, atom, nucleus | M | kilogram | kg | kg | ||||
| Mass defect | M | kilogram | kg | kg | ||||
| Elementary charge | T.I. | pendant | WITH | Cl | ||||
| Magneton nuclear | L2I | ampere square meter | A×m2 | A×m2 | ||||
| Gyromagnetic ratio | M-1TI | ampere square meter per joule second | A× m2/(J×s) | A×m2/(J×s) | ||||
| Nuclear quadrupole moment | L2 | square meter | m2 | m2 | ||||
| Binding energy, level width | L2MT-2 | joule | J | J | ||||
| Radiation intensity (energy flux density) | MT-3 | watt per square meter | W/m2 | W/m2 | ||||
| Nuclide activity (in radioactive source) | T-1 | becquerel | Bq | Bk | ||||
| Specific activity | M-1T-1 | becquerel per kilo | Bq/kg | Bq/kg | ||||
| Molar activity | M-1N-1 | becquerel per mole | Bq/mol | Bq/mol | ||||
| Volumetric activity | L-3T-1 | becquerel per cubic meter | Bq/m3 | Bq/ m3 | ||||
| Surface activity | L-2T-1 | becquerel per square meter | Bq/m2 | Bq/m2 | ||||
| Half-life, average lifespan | T | second | s | With | ||||
| Decay constant | T-1 | second to the minus first power | s-1 | s-1 | ||||
| Effective cross section | L2 | square meter | m2 | m2 | ||||
| Differential effective cross section | L2 | square meter per steradian | m7sr | m2/avg | ||||
| Mobility | M-1T2I | square meter per volt second | m2/(Vs) | m2/(W×s) | ||||
| Retarding ability of the medium | L-1 | meter to the minus first power | m-1 | m-1 | ||||
| Deceleration length, diffusion length, migration length | L | meter | m | m | ||||
APPLICATIONS
Appendix A The most common stylistic mistakes.
Appendix B Norms of Russian-language technical literature.
Appendix B Examples of translation of some frequently used terms.
Appendix D “False friends” of the translator.
Appendix E Presentation of numbers, monetary amounts and dates
Appendix E Additional requirements for translations that require notarization of the authenticity of the translator's signature.
Appendix G Features of translating descriptions of software products.
Appendix 3 Recommendations for setting up MS Word before starting translation.
Appendix I Some features of typing in foreign languages.
Appendix K Directory of units of measurement. part 2 part 3 part 4
Appendix L Transliteration rules.
translation agency translation services prices contacts vacancies articles
Table of units of measurement “Optics, electromagnetic radiation”
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| The power of light | J, I | candela | cd | The amount of light energy emitted in a given direction per unit time. | Luminous, extensive value |
| Light flow | F | lumen | lm | Physical quantity characterizing the amount of “light” power in the corresponding radiation flux | |
| Light energy | Q | lumen-second | lm•s | Physical quantity characterizes the ability of energy transferred by light to cause visual sensations in a person | |
| Illumination | E | luxury | OK | The ratio of the luminous flux incident on a small area of a surface to its area. | |
| Luminosity | M | lumen per square meter | lm/m2 | Luminous quantity representing luminous flux | |
| Brightness | L,B | candela per square meter | cd/m2 | Luminous intensity emitted per unit surface area in a specific direction | |
| Radiation energy | E,W | joule | J = (kg m2/s2) | Energy transferred by optical radiation |
Tasks for the circulation period
No. 4. The blades of a windmill rotate with a period of 5 seconds. Calculate the number of revolutions of these blades in 1 hour.
You only need to convert time to SI units for 1 hour. It will be equal to 3,600 seconds.
Selection of formulas. The period of rotation and the number of revolutions are related by the formula T = t: N.
Solution. From the above formula, the number of revolutions is determined by the ratio of time to period. Thus, N = 3600: 5 = 720.
Answer. The number of revolutions of the mill blades is 720.
No. 5. An airplane propeller rotates at a frequency of 25 Hz. How long will it take the propeller to make 3,000 revolutions?
All data is given in SI, so there is no need to translate anything.
Required formula: frequency ν = N: t. From it you only need to derive the formula for the unknown time. It is a divisor, so it is supposed to be found by dividing N by ν.
Solution. Dividing 3,000 by 25 gives the number 120. It will be measured in seconds.
Answer. An airplane propeller makes 3000 revolutions in 120 s.
Table of units of measurement "Acoustics"
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Sound pressure | p | pascal | Pa | Variable excess pressure arising in an elastic medium when a sound wave passes through it | |
| Volume velocity | c, V | cubic meter per second | m3/s | The ratio of the volume of raw materials supplied to the reactor per hour to the volume of catalyst | |
| Sound speed | v, u | meter per second | m/s | Velocity of propagation of elastic waves in a medium | |
| Sound intensity | l | watt per square meter | W/m2 | A quantity characterizing the power transferred by a sound wave in the direction of propagation | scalar physical quantity |
| Acoustic impedance | Za, Ra | pascal second per cubic meter | Pa•s/m3 | The ratio of the amplitude of sound pressure in a medium to the vibrational speed of its particles when a sound wave passes through the medium | |
| Mechanical resistance | Rm | newton second per meter | N•s/m | Indicates the force required to move a body at each frequency |
Some practical capacitor designs
In practice, various designs of flat capacitors are used. The design of the device determines its characteristics and scope of application.
Variable capacitor
A common type of variable capacitor (VCA) consists of a block of moving and fixed plates separated by air or a solid insulator. The moving plates rotate around an axis, increasing or decreasing the overlap area. When the moving block is removed, the interelectrode gap remains unchanged, but the average distance between the plates also increases. The dielectric constant of the insulator also remains unchanged. The capacity is adjusted by changing the area of the plates and the average distance between them.
KPI in the position of maximum (left) and minimum (right) capacity
Oxide capacitor
Previously, such a capacitor was called electrolytic. It consists of two strips of foil separated by a paper dielectric impregnated with electrolyte. The first strip serves as one plate, the second plate serves as the electrolyte. The dielectric is a thin layer of oxide on one of the metal strips, and the second strip serves as a current collector.
Table of units of measurement “Atomic and nuclear physics. Radioactivity"
| Physical quantity | Symbol | Unit of measurement of physical quantity | Unit change physical led | Description | Notes |
| Mass (rest mass) | m | kilogram | kg | The mass of an object at rest. | |
| Mass defect | Δ | kilogram | kg | A quantity expressing the influence of internal interactions on the mass of a composite particle | |
| Elementary electric charge | e | pendant | Cl | The minimum portion (quantum) of electric charge observed in nature in free long-lived particles | |
| Communication energy | Est | joule | J = (kg m2/s2) | The difference between the energy of a state in which the constituent parts of the system are infinitely distant | |
| Half-life, average lifetime | T, τ | second | With | The time during which the system decays in the approximate ratio of 1/2 | |
| Effective cross section | σ | square meter | m2 | A quantity characterizing the probability of interaction of an elementary particle with an atomic nucleus or another particle | |
| Nuclide activity | A | becquerel | Bk | A value equal to the ratio of the total number of decays of radioactive nuclide nuclei in the source to the decay time | |
| Energy of ionizing radiation | E,W | joule | J = (kg m2/s2) | Type of energy released by atoms in the form of electromagnetic waves (gamma or x-rays) or particles | |
| Absorbed dose of ionizing radiation | D | gray | Gr | The dose at which 1 joule of ionizing radiation energy is transferred to a mass of 1 kg | |
| Equivalent dose of ionizing radiation | H , Deck | sievert | Sv | Absorbed dose of any ionizing radiation equal to 100 erg per 1 gram of irradiated substance | |
| Exposure dose of X-ray and gamma radiation | X | pendant per kilogram | C/kg | ratio of the total electric charge of ions of the same sign from external gamma radiation |
Formulas containing capital N
The first of them determines power, which is equal to the ratio of work to time:
N = A: t.
In molecular physics there is such a thing as the chemical amount of a substance. Denoted by the Greek letter "nu". To count it, you need to divide the number of particles by Avogadro's number:
ν = N:NA.
By the way, the last value is also denoted by the so popular letter N. Only it always has a subscript - A.
To determine the electric charge, you will need the formula:
q = N × e.
Another formula with N in physics is oscillation frequency. To count it, you need to divide their number by time:
ν = N : t.
The letter “en” appears in the formula for the circulation period:
T = t: N.