Chemical current sources (abbreviated CIT) are devices in which the energy of a redox reaction is converted into electrical energy. Their other names are electrochemical cell, galvanic cell, electrochemical cell. The principle of their operation is as follows: as a result of the interaction of two reagents, a chemical reaction occurs with the release of direct electric current energy. In other current sources, the process of generating electricity occurs according to a multi-stage scheme. First, thermal energy is released, then it turns into mechanical and only then into electrical energy. The advantage of HIT is that the process is one-stage, that is, electricity is obtained immediately, bypassing the stages of obtaining thermal and mechanical energy.
Chemical current sources
Category: Household goods
Chemical current sources are devices in which the chemical energy of the active substances contained in them is converted into electrical energy during electrochemical reactions. They are used for autonomous power supply of small-sized electronic equipment, watches, mobile phones, video cameras and for local lighting.
Chemical current sources are divided into primary and secondary.
The primary chemical sources of current are galvanic cells and batteries intended for one-time continuous or intermittent discharge.
A galvanic cell is a chemical current source consisting of electrodes with different electrical potentials and an electrolyte enclosed in one vessel.
A galvanic battery is a chemical current source consisting of two or more galvanic cells electrically connected to each other.
- Secondary chemical current sources include batteries intended for repeated use by recharging with electric current.
- According to the material of the electrodes, chemical current sources are divided into manganese-zinc, mercury-zinc, lithium-ion (Li-ion), copper-lithium, nickel-cadmium (Ni-Cd), nickel-metal hydride (Ni-MH) and etc.
- According to the shape of the body, chemical current sources are cylindrical, prismatic and disk.
- The main parameters of chemical current sources are: capacity, open circuit voltage, charge retention.
- The capacity of a chemical current source is a value corresponding to the amount of electricity in ampere-hours (Ah) that a chemical current source can deliver when discharged from the initial to the final voltage.
During storage, chemical current sources lose energy due to spontaneous processes occurring in them. In this case, they talk about self-discharge of a chemical current source. The time during which a chemical current source maintains its parameters within established limits, subject to storage conditions, is called the shelf life.
Each cell and battery must indicate: product designation; manufacturer's trademark; date of manufacture (month and year); Rated voltage; warranty period of storage.
Markings and external non-metallic coatings of elements must be resistant to the effects of alcohol-gasoline mixtures. It is recommended to store chemical sources in the temperature range from 10°C to minus 20°C.
Secondary power sources or batteries
In batteries, when electric current from an external circuit (charge) is passed through them, chemical reactions occur in the electrodes and solutions that are close to circulating, and the work of the electric current is accumulated in the form of free energy of the reaction products. The battery produces an electric current when discharged, after which it can be charged again.
Figure 2. General battery design
The most widely used are acid lead, alkaline cadmium-nickel and alkaline silver-zinc batteries.
3.1. Lead acid battery
A lead acid battery in a charged state is an element:
(-) Pb, PbSO4 (t) | H2SO4 (32-34%) | PbO2, Pb (+)
during operation of which the following electrode reactions occur:
on the left cathode:
on the right electrode:
total reaction of the process:
Figure 3. Lead-acid battery operation diagram
The isobaric potential of this reaction reflects the disappearance of solid lead and its dioxin, the appearance of solid lead sulfate, as well as the disappearance of 2 moles of sulfuric acid and the appearance of 2 moles of water:
Since the chemical potentials of solid phases are constant (at a given temperature and pressure), then
The isobaric potential and emf of the battery depend on the concentration of sulfuric acid (more precisely, on the activity of the components of the solution). As the battery discharges, the concentration of sulfuric acid decreases, and when charging it increases.
2.3.2 Nickel-cadmium alkaline batteries This battery, when charged, is an electrochemical cell:
(-)Cd | Cd (OH) 2, KOH (20%) | | KOH (20%), Ni (OH) 2, Ni (OH) 3/Ni (+)
Total reaction for this element:
Cd + 2Ni (OH) 3 = Cd (OH) 2 + 2Ni (OH) 2
The value for this reaction should not depend on the alkali concentration, since only solids participate in the overall reaction. However, reactions on the electrodes are accompanied by a change in the alkali concentration and the formation of a concentration difference in the two electrodes:
This difference should determine the concentration polarization, which reduces the emf of the element. However, as a result of stirring under conditions of proximity of the electrodes, this difference in concentrations practically does not arise. The emf of a cadmium-nickel battery is approximately 1.36 V. The following alkaline batteries are used, in which cadmium and cadmium oxide are replaced by iron and ferrous iron.
2.3.4 silver - zinc alkaline batteries The anode is a porous zinc plate, the cathode is silver oxides Ag2O and AgO, obtained by electrolytic oxidation of metallic silver. The electrolyte is a concentrated solution of KOH, saturated with potassium zincates Zn (OK) 2. The battery can be represented as:
(-) Zn | Zn (OK) 2 + KOH (40%) | Ag2O or AgO | Ag(+)
The total reaction in this element is AgO + Zn = ZnO + Ag
The process takes place in two stages: AgO is reduced first to Ag2O, then to metallic silver. The EMF of elements with an AgO cathode is 1.86 V, with an Ag2O cathode - 1.58-1.60 V. At low current density, the voltage drops by 0.3 V when moving from the first stage to the second. In practice, only the second stage is used. After battery discharge:
(-) Zn | ZnO, Zn (OK) 2 + KOH (40%) | Ag(+)
In such batteries, unlike lead and alkaline batteries, the electrolyte does not participate in charge and discharge reactions, so it can be taken in small quantities. This made it possible to design batteries that have a very efficient design: the electrodes are located next to each other and separated by a thin layer of cellophane. All the electrolyte is located in the pores of the electrodes. Silver-zinc batteries have a large capacity, high energy and high power per unit of mass and volume, which is why they are widely used where small batteries are needed.
OPTION
RESULTS ° ROCK Ñозда OPTIONS OPTIONS µ и Ñ Ð¼ÐµÐ½ÑÑими линейнÑми ÑазмеÑами. RESULTS в, вÑп¾Ð»Ð½ÐµÐ½Ð½ÑÑ Ð¿Ð¾ линейнÑм ÑÑемам. RESULTS ´Ð¾ знаÑÐµÐ½Ð¸Ñ 98%. RESEARCH ºÑоÑÑемÑ, вÑполнÑÑÑие ÑÑнкÑии конÑÑоллеÑов.
RESULTS ROOM RESULTS. она веÑÑма многообÑазна. RESULTS Sorry. RESULTS RESULTS ¼Ð °Ð»Ð¾Ðµ знаÑение ÑÑÐ¾Ð²Ð½Ñ ÑобÑÑвенного ÑÑма. RESULTS SMALLERY ¿Ð¾Ð¼ÐµÑ в ÑÐ ¸Ñоком ÑаÑÑоÑном диапазоне. RESULTS Ò ¸Ð¼Ð¸, ÑÑо пÑÐ¸Ð²Ð¾Ð´Ð¸Ñ Ðº ÑдоÑÐ¾Ð¶Ð°Ð½Ð¸Ñ Ð¸Ð·Ð´ÐµÐ»Ð¸Ð¹, в RESULTS.
Classification of electric current sources
The table of electric current sources presents the main types of sources and the mechanisms of their operation.
| Electric current source | Mechanism for separating electrical charges |
| Electrophore machine | Mechanical rotational energy |
| Thermoelements | Thermal energy |
| Solar batteries, photocells | Photon energy of light |
| Galvanic cells, batteries | Chemical reactions |
| Batteries | Chemical reactions |
| Electromagnetic generators | Mechanical rotational energy |
Attempts are constantly being made to harness human mechanical energy to generate electricity. For example, a version of a jump rope was proposed that had cavities inside the cylindrical handle. They contain batteries. According to calculations, 20-25 jumping ropes will charge four batteries.
Design and principle of operation of HIT
The device of chemical current sources includes two electrodes (conductors of the first kind) and an electrolyte located between them (conductor of the second kind, or ionic conductor). An electronic potential arises at the boundary between them. The electrode on which the oxidation of the reducing agent occurs is called the anode, and the one on which the oxidizing agent is reduced is called the cathode. Together with the electrolyte they form an electrochemical system.
A byproduct of the redox reaction between the electrodes is the generation of electric current. During such a reaction, the reducing agent is oxidized and gives electrons to the oxidizing agent, which accepts them and is thereby reduced. The presence of an electrolyte between the cathode and anode is a necessary condition for the reaction. If you simply mix powders from two different metals, no electricity will be released; all the energy will be released in the form of heat. An electrolyte is needed to streamline the electron transfer process. Most often, it is a saline solution or a melt.
Electrodes look like metal plates or grids. When they are immersed in an electrolyte, a difference in electrical potential arises between them - an open circuit voltage. The anode tends to give up electrons and the cathode tends to accept them. Chemical reactions begin on their surface. They stop when the circuit is opened, as well as when one of the reagents is used up. The circuit opens when one of the electrodes or electrolyte is removed.
Lithium "plus"
The functioning of lithium-ion batteries is based on the ability of materials with a certain structure (the so-called “matrix”) to reversibly incorporate lithium ions. Such matrices act as a “host”, which provides free spaces in its structure to the “guest” – the lithium ion Li+. During the process of charging (discharging) the battery, these ions leave one matrix and are introduced into another. The output electrical voltage of such systems is slightly less than that of lithium metal systems, but the level of safety is significantly higher.
In terms of basic technical characteristics, LIBs are significantly superior to their “competitors”. Thus, compared to nickel-metal hydride analogs, LIBs have twice the electrochemical capacity and almost three times the accumulated energy density and power density. LIB can withstand very high discharge currents, which is important for use in powerful portable power tools. Self-discharge is only 2-5%, and the number of charge-discharge cycles without loss of capacity is 4-6 times higher than that of their predecessors. LIBs are also less susceptible to the so-called memory effect - they can begin to be recharged at any time, without waiting for complete discharge.
Electrochemistry as a science that studies the relationship between electrical phenomena and chemical reactions began with the experiments of the Italian L. Galvani at the end of the 18th century, who discovered that touching metal objects to muscle tissue causes the same effect (sharp contraction) as the action of a discharge from the Leiden banks" - the very first energy storage device. Galvani's famous compatriot, A. Volta, suggested that the “galvanic” effect was due to the presence of contact between dissimilar metals, and in 1800 he created a device that produced an “inexhaustible charge.” The Voltaic Column became the world's first chemical source of current (before that, the only sources of electricity were machines that generated static electricity through friction). In this source there was a direct conversion of chemical energy into electrical energy. Over the next two decades, the electrolytic decomposition of water into hydrogen and oxygen, as well as the electrodeposition of metals from solutions, was carried out. By electrolysis of molten salts, the outstanding English scientist H. Davy isolated alkali metals, including lithium, in their pure form. With the help of chemical current sources, a number of important physical discoveries were made, including the phenomenon of the magnetic action of electric current (Ampere, 1820), the law of proportionality of current and voltage (Ohm, 1827), heat generation during the passage of current (Joule, 1843), electromagnetic induction (Faraday, 1931). And the Russian scientist B. Jacobi, who designed the first electric motor back in 1834, four years later created the most important practical application of electrochemistry - galvanoplasty
But LIB also has disadvantages, first of all, a high risk of explosive destruction when overcharged or overheated. Therefore, all household batteries have an electronic circuit built into them that limits the charging voltage. In addition, LIBs are completely destroyed as a result of deep discharge, and in general these batteries are still quite expensive.
However, it should be noted that lithium-ion technologies are only at the beginning of their journey, while their “competitors” are very close to their theoretical limit. Having already been introduced into industrial production, LIBs are still the subject of intensive study aimed at improving their electrochemical characteristics. All three components of the system are being improved: electrolyte, cathode and anode.
Chemical sources of electric current
Chemical current sources are devices whose operation is determined by the conversion of chemical energy released during the redox process into electrical energy.
The advantages of chemical power sources include the versatility of their use.
The power source for many household devices, as well as devices used in scientific laboratories or in production, are chemical power sources.
The demand for chemical current sources in ensuring the functioning of communication equipment or portable electronic equipment deserves special attention, since in this case they are irreplaceable.
Chemical sources of electric current
Structurally, chemical current sources consist of two metal electrodes separated by an electrolyte. Electrodes are made of a metal, which is a conductor of electrons (electronic conductivity), and an electrolyte is made of a liquid or solid, which is a conductor of ions (ionic conduction).
If high voltage is required to power any consumer, then the electric batteries are connected in series. In cases where high current is required for power supply, electric batteries are connected in parallel and are called a battery.
Series connection (consistent connection)
Total = E1 + E2 + E3
Mixed connection (counter)
Total = E1 – E2 + E3
- Parallel connection of power supplies. (This connection is used
- to increase the current in the circuit. )
Total = E1 = E2 = E3
Depending on the nature of the work, various types of chemical power sources are called galvanic cells or electric batteries.
A distinctive feature of chemical current sources, called galvanic cells, is the possibility of one-time use, since their active substances that release electrical energy are subject to complete disintegration during a chemical reaction. When a galvanic cell is completely discharged, its further use is impossible.
A feature of such chemical current sources as electric batteries is their reusable use due to the reversibility of the main operating processes.
A discharged electric battery has the ability to regenerate its electrical energy-producing active substances through the process of passing direct current through it, the source of which is another device.
When charging an electric battery, the direct current from another source must flow in the direction opposite to the discharge current. This condition promotes the replacement of the oxidation reaction with a reduction reaction on the positive electrode, and vice versa, on the negative electrode the oxidation reaction is replaced with a reduction reaction.
A number of general and special technical requirements are imposed on chemical current sources. All requirements are specified in the relevant regulatory documentation.
The general requirements are: for overall dimensions and weight characteristics; to reliability; to the absence of harmful effects on the environment; for safe use by maintenance personnel; to service life; to minimum self-discharge.
Special technical conditions include requirements for specific characteristics, mechanical strength, operating temperature range, low internal resistance, operability in any position, and ease of use.
Classification of galvanic cells and their selection
Basic concepts about relay protection
Generators of electric current resulting from a chemical reaction are divided into:
- Sizes;
- Design features;
- The method and reagent through which electricity is generated.
All elements that produce current during a chemical reaction are divided into:
- Chargers that can be repeatedly charged from a direct current source during operation are called batteries;
- Non-rechargeable, that is, disposable sources that, after the completion of a chemical reaction, simply become unusable and must be disposed of. Simply it is a galvanic cell or battery.
In order to select a source of electricity based on a chemical reaction, you need to understand its characteristics, which include:
- Voltage between anode and cathode when the circuit is open. This indicator most often depends on the selected electrochemical system, as well as the concentration and treated of all components;
- Source power;
- Current indicator;
- Capacity;
- Electrical indicators, that is, the number of charge and discharge cycles;
- Operating temperature range;
- The shelf life between the time an item is created and before it is put into use;
- Full service life;
- Strength, that is, protection of the housing from various mechanical damage and influences, as well as vibrations;
- Working position, some of them only work in horizontal positions;
- Reliability;
- Easy to operate and maintain. Ideally, there is no need for the slightest intervention in the work during the entire service life.
When choosing the right battery or accumulator, you must take into account its electrical ratings such as voltage and current, as well as capacity. It is this that is key to maintaining the functionality of the device connected to the source.
Operating principle
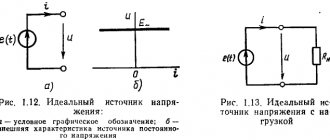
The basis of chemical current sources are two electrodes (a negatively charged anode containing a reducing agent and a positively charged cathode containing an oxidizing agent) in contact with the electrolyte. A potential difference is established between the electrodes - an electromotive force corresponding to the free energy of the redox reaction. The action of chemical current sources is based on the occurrence of spatially separated processes in a closed external circuit: at the negative anode, the reducing agent is oxidized, the resulting free electrons pass through the external circuit to the positive cathode, creating a discharge current, where they participate in the reduction reaction of the oxidizing agent. Thus, the flow of negatively charged electrons through the external circuit goes from the anode to the cathode, that is, from the negative electrode (the negative pole of the chemical current source) to the positive. This corresponds to the flow of electric current in the direction from the positive pole to the negative, since the direction of the current coincides with the direction of movement of positive charges in the conductor.
Modern chemical current sources use:
- as a reducing agent (anode material) - lead Pb, cadmium Cd, zinc Zn and other metals;
- as an oxidizing agent (cathode material) - lead(IV) oxide PbO2, nickel hydroxide NiOOH, manganese(IV) oxide MnO2 and others;
- as an electrolyte - solutions of alkalis, acids or salts.
ROOM
RESULTS ROOM вание линейнÑÑÑ. RESULTS CONTACT US е — ÑнаÑала пеÑеменное напÑÑжение вÑпÑÑмР¸Ñелем пÑеобÑазÑеÑÑÑв поÑÑÑоÑнное, заѵм вÑÐ ° RESULTS the ½Ð¾Ð¼ каÑкаде Ñнова RESULTS ok.
RESPONSIBILITY 15-60 ) кÐÑ . ROOM RESULTS ¼Ð¾Ð´ÑлÑÑии ( ШÐÐ), пÑи коÑоÑой ÑÑÐ¾Ð²ÐµÐ½Ñ Ñигнала на вÑоде RESULTS ROOM и знаÑением Ð¸Ñ ÑкважноÑÑи. ROOM 1/2 "ÑзÑÑÑÑÑ Ð¿Ñи Ñоздании ап¿¿Ð°ÑаÑÑÑÑÑÑазÐ"иÑного н азнаÑениÑ.
History of creation
Voltaic pole
The first chemical current source was invented by the Italian scientist Alessandro Volta in 1800. It was a “Volta element” - a vessel with salt water with zinc and copper plates lowered into it, with wire current leads. The scientist then assembled a battery from these elements, which was later called a “voltaic column.” This invention was subsequently used by other scientists in their research. For example, in 1802, Russian academician V.V. Petrov constructed a voltaic column of 2100 elements to produce an electric arc. In 1836, English chemist John Daniel improved the Voltaic element by placing zinc and copper electrodes in a solution of sulfuric acid. This design became known as the “Daniel element.”
In 1859, French physicist Gaston Plante invented the lead-acid battery by placing a thin rolled-up lead plate in sulfuric acid. This type of cell is still used in car batteries today.
In 1865, the French chemist J. Leclanchet proposed his galvanic cell (Leclanchet element), which consisted of a zinc cup filled with an aqueous solution of ammonium chloride or other chloride salt, into which an agglomerate of manganese(IV) oxide MnO2 was placed as a depolarizer with a carbon current drain. . A modification of this design is still used in salt batteries for various household devices.
In 1890, in New York, Conrad Hubert, an immigrant from Russia, creates the first pocket electric flashlight. And already in 1896, the National Carbon company began mass production of the world's first dry cells, Leclanche "Columbia".
The oldest voltaic cell still in use is the silver-zinc battery, manufactured in London in 1840. Connected to two such batteries in series, a bell still operates to this day in the Clarendon Laboratory at Oxford.
History of creation
Voltaic pole
The first chemical current source was invented by the Italian scientist Alessandro Volta in 1800. It was a “Volta element” - a vessel with salt water with zinc and copper plates lowered into it, with wire current leads. The scientist then assembled a battery from these elements, which was later called a “voltaic column.” This invention was subsequently used by other scientists in their research. For example, in 1802, Russian academician V.V. Petrov constructed a voltaic column of 2100 elements to produce an electric arc. In 1836, English chemist John Daniel improved the Voltaic element by placing zinc and copper electrodes in a solution of sulfuric acid. This design became known as the “Daniel element.”
In 1859, French physicist Gaston Plante invented the lead-acid battery by placing a thin rolled-up lead plate in sulfuric acid. This type of cell is still used in car batteries today.
In 1865, the French chemist J. Leclanchet proposed his galvanic cell (Leclanchet element), which consisted of a zinc cup filled with an aqueous solution of ammonium chloride or other chloride salt, into which an agglomerate of manganese(IV) oxide MnO2 was placed as a depolarizer with a carbon current drain. . A modification of this design is still used in salt batteries for various household devices.
In 1890, in New York, Conrad Hubert, an immigrant from Russia, creates the first pocket electric flashlight. And already in 1896, the National Carbon company began mass production of the world's first dry cells, Leclanche "Columbia".
The oldest voltaic cell still in use is the silver-zinc battery, manufactured in London in 1840. Connected to two such batteries in series, a bell still operates to this day in the Clarendon Laboratory at Oxford.
Modern chemical current sources and their applications
It is difficult for a person to live a modern life without these mobile energy generators, which he encounters throughout his life, starting with children's toys and ending with, say, a car.
The areas of application of various batteries and accumulators are so diverse that it is very difficult to list them. The operation of any mobile phone, computer, laptop, watch, remote control would be impossible without this portable and very compact device for creating a stable electrical charge. In medicine, sources of chemical energy are widely used to create any device that helps a person live a full life. For example, for hearing aids and pacemakers that can only operate from portable voltage sources, so as not to confine a person to wires. In production, entire battery systems are used to provide voltage for shutdown and protection circuits in the event of a loss of incoming high voltage at substations. And this food is also widely used in all vehicles, military and space technology. One of the types of common batteries is lithium electric current sources, since this particular element has a high specific energy. The fact is that only this chemical element turns out to have a strong negative potential among all substances known and studied by man. Lithium-ion batteries stand out among all other batteries in terms of the amount of energy generated and their small dimensions, which allows them to be used in the most compact and small electronic devices.
Methods for recycling chemical energy sources
The problem of recycling chemical voltage sources of different sizes is an environmental problem for the entire planet. Modern sources contain up to thirty chemical elements that can cause significant harm to natural resources, therefore entire programs have been developed for their disposal and specialized processing workshops have been built. Some methods make it possible not only to efficiently recycle these harmful substances, but also to return them to production, thereby protecting the environment. In order to extract non-ferrous metals from batteries and accumulators, entire pyrometallurgical and hydrometallurgical complexes have now been developed and used in civilized countries that monitor and care about the environment. The most common method for recycling waste chemical power sources is a method that works by combining these processes. Its main advantage is considered to be a high degree of extraction with a minimum amount of waste. This method of pyrometallurgical, hydrometallurgical and mechanical processing includes eight main stages:
- Grinding;
- Magnetic separation;
- Burning;
- Additional grinding;
- Isolation of large and small elements using screening;
- Water purification and leaching;
- Sulfuric acid leaching;
- Electrolysis.
Organizing the correct collection and disposal of chemical waste makes it possible to minimize the negative impact on both the environment and human health.
Operating principle
The basis of chemical current sources are two electrodes (a positively charged anode containing a reducing agent and a negatively charged cathode containing an oxidizing agent) in contact with the electrolyte. A potential difference is established between the electrodes - an electromotive force corresponding to the free energy of the redox reaction. The action of chemical current sources is based on the occurrence of spatially separated processes in a closed external circuit: at the negative anode, the reducing agent is oxidized, the resulting free electrons pass through the external circuit to the positive cathode, creating a discharge current, where they participate in the reduction reaction of the oxidizing agent. Thus, the flow of negatively charged electrons through the external circuit goes from the anode to the cathode, that is, from the negative electrode (the negative pole of the chemical current source) to the positive. This corresponds to the flow of electric current in the direction from the positive pole to the negative, since the direction of the current coincides with the direction of movement of positive charges in the conductor.
Modern chemical current sources use:
- as a reducing agent (anode material) - lead Pb, cadmium Cd, zinc Zn and other metals;
- as an oxidizing agent (cathode material) - lead(IV) oxide PbO2, nickel hydroxide NiOOH, manganese(IV) oxide MnO2 and others;
- as an electrolyte - solutions of alkalis, acids or salts.
Story
How did the first current sources appear? The chemical sources are called galvanic cells in honor of the eighteenth-century Italian scientist Luigi Galvani. He was a doctor, anatomist, physiologist and physicist. One of the areas of his research was the study of animal reactions to various external influences. The chemical method of generating electricity was discovered by Galvani by accident, during one of his experiments on frogs. He attached two metal plates to the exposed nerve in the frog's leg. At the same time, a muscular contraction occurred. Galvani's own explanation of this phenomenon was incorrect. But the results of his experiments and observations helped his compatriot Alessandro Volta in subsequent research.
Volta outlined in his writings the theory of the generation of electric current as a result of a chemical reaction between two metals upon contact with the muscle tissue of a frog. The first chemical current source looked like a container with a saline solution, with zinc and copper plates immersed in it.
HITs began to be produced on an industrial scale back in the second half of the nineteenth century, thanks to the Frenchman Leclanche, who invented the primary manganese-zinc cell with a salt electrolyte, named after him. A few years later, this electrochemical cell was improved by another scientist and was the only primary chemical source of current until 1940.
Where are they used?
It seems that the current generated as a result of chemical energy is minimal and can only be used to make ordinary batteries for a player or watch. But that's not true. The electricity obtained in this way is used in the following areas:
- transport;
- space;
- medical;
- in simple life.
Due to their design and operating principle, such devices are universal and can be used in many areas and industries.
Note! The most popular currently is the chemical battery, which is used in everyday life and industry, powering a variety of instruments and devices. Batteries for electronics and cars are also used in everyday life.
Electricity is a vital resource for modern humanity. You can get electricity thanks to certain current sources, but the most popular and convenient are chemical ones. Additionally, they are considered very environmentally friendly to use if disposed of correctly.
A little history of the creation of HIT
Back in the eighteenth century, the Italian scientist Luigi Galvani came up with the simplest element that chemically generated electric current. However, he was not only a scientist, but also a physicist, doctor, and physiologist. He was interested in and conducted experiments that were aimed at studying the reactions of animals to external stimuli. Like everything ingenious, the first chemical source of energy was obtained by Luigi absolutely by accident, during numerous experiments on frogs. After attaching two metal plates to the frog's leg muscle, muscle contraction was observed. Galvani considered this a nervous reaction to an external stimulus and outlined this in the results of his research, which fell into the hands of another great scientist, Alessandro Volta. He laid out his theory about the occurrence of tension as a result of a chemical reaction that arose between two metal plates in the environment of the muscle tissue of a frog.
The first chemical source of electric current was a container with a salt composition, into which two plates of different materials were immersed. One is copper, the other is zinc. It was this device that in the future, and more specifically in the second half of the nineteenth century, was used in the invention and creation of a manganese-zinc cell within which was the same salt electrolyte.
Again about online application generators
In the previous article in this series, we discussed creating applications for the Android platform using Java and Android Development Tools (ADT). Today we will continue this topic by getting acquainted with a new product from Embarcadero - RAD Studio XE 5. Essentially, this product is a modern incarnation of Delphi, a Windows application development tool that has been incredibly popular in our country for many years, starting from the first 16 bit version, which appeared in the second half of the 1990s. But, unlike Delphi of the late 1990s and early 2000s, RAD Studio XE 5 is a tool that allows you to create applications not only for Windows, but also for Mac OS, Android, iOS, and, importantly, based on one and the same source code and resources - having created an application project, you can simply compile the executable code for any of the named platforms. Development of mobile applications is possible using the Architect, Ultimate, Enterprise editions, as well as the Professional edition, provided that the Mobile Add-On Pack extension module is purchased with it. The development environment itself is available only for the Windows platform. If desired, in RAD Studio XE 5 you can develop in C++Builder, as well as create HTML-5 applications.
This publication cannot be considered a traditional product review - like all articles in this series, it gives an idea of the tool by describing the process of creating a mobile application with minimal effort.
You can get started with RAD Studio XE 5 by downloading a 30-day trial from Embarcadero (available for various versions of Windows). The product installation process is carried out directly from the manufacturer’s website, so it is worth doing this by ensuring that you have a reliable and not too slow Internet connection.
After installing RAD Studio XE 5 itself, you should take care of installing support tools for the platforms for which you intend to create the application. So, to develop applications for the Android platform, we need the Android SDK, already familiar to us from the previous article, with support for selected versions of the platform - it can be installed by launching the Android Tools application included with RAD Studio XE 5 (Fig. 1).
Rice. 1. Selecting an application for modification
If the application is to be tested on a real device, its driver should be installed on the computer with the development environment (usually it can be found either on the device manufacturer’s website or on the Google website). On the device itself, you should enable remote debugging of applications in the settings.
To develop iOS applications, you will need a computer running Mac OS X to run the emulator. If you don’t have the latter, you can use Embarcadero’s MacinCloud cloud service, which also has a trial period of 24 hours. To test iOS applications on mobile devices and then upload them to the AppStore, you should also join the Apple iOS Developer Program or a similar program for universities or corporate clients.
The development environment itself looks quite traditional (Fig. 2) - a project manager (Project Manager, top right), a component palette with groups of interface elements and so-called non-visual components (Tool Palette, bottom right), a tree with the structure of form components (Structure, left top), Object Inspector (bottom left), form and code editor (center).
Rice. 2. Replacing the application logo
We will begin creating our very first application (as always, dedicated to introducing ComputerPress magazine) by using a ready-made template. To do this, in the main menu of the development environment, select the item File->New->FireMonkey Mobile Application and, among the proposed application templates, select the element Tabbed with Navigation (Fig. 3).
Rice. 3. Editing buttons
As a result, an application project will be generated, similar to the one the creation of which was described in the previous article of this series, and which is a notepad with four tabs, on each of which you can further place interface elements (Fig. 4).
Rice. 4. Setting the functionality of the buttons
By selecting a potential platform for running an application in the upper right corner of the form editor, you can see how it will look when executed on a particular platform.
Next, by selecting interface elements with the mouse (in our case, labels and tab titles), we can change the labels on them using the Text property in the object inspector.
For iOS applications, using the StyleLookup property, you can also select icons placed on notepad shortcuts (Fig. 5).
Fig.5. Widgets available for selection
The next step in creating our application is to add interface elements to the notepad tabs. To do this, click on the “About the Journal” tab, select the TMemo component in the Standard group on the component palette (Delphi users are familiar with it, but for beginners I’ll tell you that it’s just a multi-line text editor) and place it on the form. Let's find the Strings property in the object inspector, click on the button with the ellipsis and copy the brief information about our journal borrowed from our website www.compress.ru into the property editor that opens. From the list of possible values for the Align property of our Memo1 component, we select the value alClient - in this case, our text editor will occupy the entire area of the screen between the title and labels. And finally, we will set the Enabled property of this component to False so that the user of our application cannot edit the text (Fig. 6).
Rice. 6. Publish the application for testing
Let’s repeat all the same steps with the “Categories” tab, placing in the created Memo2 component the text borrowed from the same website about the magazine’s headings.
Next, let's give our application some variety - on the next two tabs we will place not a text editor, but a browser - the TWebBrowser component (it can be found in the Internet group of the component palette). Let's change the Align property of these components to the alClient value. Next, fill in their URL properties. For the component on the “Our Site” tab, enter https://www.compress.ru as the value of this property, and for the TWebBrowser component on the “Coordinates” tab, enter a link to the Yandex.Maps server, which will indicate the search result for the building editorial office on the map of Moscow. Then save the project.
So far, our application has certain disadvantages: for example, it does not have the ability to return to the previous page in browsers on the third and fourth tabs. Let's correct this shortcoming. To do this, at the top of one of the browser tabs, we will place two TSpeedButton buttons, linking one of them to the upper left and the other to the upper right corner by selecting the options akTop, akLeft, akRight of the Anchors property of each button and adding arrow images to them using the already familiar StyleLookup property (Fig. 7).
Rice. 7. Finished application - iPhone 5 screenshots
Let's double-click on each of the buttons, after which empty event handlers associated with clicking on them will be generated. Let's add one line of code to them (highlighted in bold):
procedure TTabbedwithNavigationForm.SpeedButton1Click(Sender: TObject);
begin
WebBrowser1.GoForward;
end;
procedure TTabbedwithNavigationForm.SpeedButton2Click(Sender: TObject);
begin
WebBrowser1.GoBack;
end;
These two lines of code mean that when you click on one of the buttons, you move to the previous page that opened in the browser, and when you click on the second button, you move to the next one.
And finally, select both buttons, copy them to the clipboard, go to the tab with the second browser and remove them from the clipboard. If you do this carefully, the buttons will be copied along with their properties and event handlers. Let's save the project.
Actually, this will complete the creation of the application. Now it can be compiled and tested. Let's start by compiling the application for the iOS platform. First, you need to connect to a computer with Mac OS X, located on the same network as the Windows computer on which development is carried out, or to the MacinCloud service. In the first case, you also need to install the Platform Assistant console application on a computer running Mac OS X and launch it, and then enter a password to access it in the RAD Studio XE 5 development environment. Then you should describe the connection to this computer by selecting the option in the main menu of the development environment Tools->Options. The Connection Profile Manager section of the Options dialog panel contains a list of connection profiles to which you can add a new profile by specifying the computer name and password to access the running instance of the Platform Assistant application (Figure 8).
Rice. 8. E-book created using the appropriate template
You can test the connection using the Test Connection button. If problems arise, it is worth checking whether the computer running the Platform Assistant application is accessible on the network and whether the port through which the connection between computers is made is open.
After successfully connecting to the Platform Assistant application, in the project manager, select the appropriate platform and the configured connection profile with a computer running Mac OS (Fig. 9).
Rice. 9. Create an iPad application
Select the main menu option Run->Run or press the F9 key. We wait for a while and an iPhone emulator with our application will appear on the screen of a computer running Mac OS X.
Now let's try to compile the same application to run on the Android platform. Let's connect the device used for testing, select the device and platform in the Target Platforms section of the project manager and run the application again using the F9 key.
After a relatively short period of time, our application will launch on the connected device.
So, we have created a cross-platform application that can run on both iOS and Android. At the same time, we did not need to use any special execution environments or code generation with subsequent compilation in development environments like Xcode or Eclipse + ADT - RAD Studio XE 5 creates so-called native applications. This means that the performance of such applications should be comparable to the performance of applications created, for example, using Xcode or ADT.
Miracles, of course, do not happen - the size of executable files created using RAD Studio XE 5 will probably be larger than when using Xcode or ADT. But space on mobile devices, you see, is not the most expensive resource today - investments in the knowledge and skills of mobile application developers will cost much more. And here RAD Studio XE 5 is still out of competition - supporting two of the most popular mobile platforms at once means that there is no need to have separate development teams for iOS and Android. It is also important that companies that decide to implement RAD Studio XE 5 have access to a huge number of Delphi and C++Builder experts of all ages and with a wide variety of experience on the Russian labor market - after all, this tool is, after all, almost 20 years old.
In conclusion, I would like to draw your attention to the fact that the Embarcadero website has a huge amount of educational materials on developing mobile applications using RAD Studio XE 5, and this collection of materials is constantly growing. Therefore, mastering this tool will not be too difficult even for beginners.
The previous two articles in this series were devoted to a brief introduction to professional application development tools using the Android Development Toolkit and a new product from Embarcadero - RAD Studio XE 5. We conclude the topic of professional tools for creating mobile applications, and today we will return to online application generation services , the use of which, as we already know, in some cases can be more effective than independent development using professional tools.
The service that today's article is devoted to is called iBuildApp and is an online application builder for smartphones that does not require any programming skills from the user. It belongs to the American company of the same name with a Russian representative office in Vladimir.
You can create applications using the iBuildApp service for free, and you can (but not necessarily) publish them in online application stores on your own. At the same time, the owners of the service provide users with a number of paid services, such as publishing applications in online stores for those who are not ready to publish on their own, assistance in monetizing applications (that is, making a profit by placing advertising in them), sending push and GPS messages -notifications to application users, 24/7 email support. Payment for services is possible on a monthly basis in accordance with various tariff plans, differing in the number of application downloads and the availability of the services listed above. Moreover, all tariff plans, including the free one, include access to ready-made application templates (including free and paid ones) and built-in components (widgets), technical support, the ability to see application performance statistics and downloads.
Special mention should be made of application templates and ready-made widgets - this service has a lot of them. Templates are available for different types of companies, types of activities, application categories, and you can change design options for a ready-made template and add widgets to it. If you wish, you can create your own templates and widgets and place them in the iBuildApp tools catalog, or order the development of a widget or even an entire application from the owners of this service.
Let's take a look at what you can create with the free plan. Let's start with the ComputerPress application, traditional for our cycle, with information about our magazine. To do this, you need to choose either a template or an almost finished application that can be modified.
From the ready-made applications, we will choose “Small Business” - the editorial offices of magazines for the most part belong to this category of companies (Fig. 1).
After making the selection, we will find ourselves in the application editor, in which, by opening the elements of the list of possible actions sequentially or in random order, we can customize its appearance or functionality, for example, replace the background or logo (Fig. 2).
I would like to draw attention to the support for the Russian application interface and the presence of a Russian interface in the online application editor - now not all such services have even the first of these features.
Next, you can make changes to the number and appearance of buttons, and you can choose your own graphic images for them (Fig. 3).
The button functionality available by default can also be replaced with the desired one. In particular, it is possible to select the necessary widgets from a fairly extensive list and configure their parameters, for example, entering text and pictures in the case of a widget displaying a static HTML document (Fig. 4).
Among the widgets available today are widgets for displaying RSS feeds, HTML documents, interfaces with Twitter and Facebook, video lists from YouTube and Vimeo, interfaces with the phone, email client, Google Map mapping services, contact management tool, calculator, camera, photo gallery, an electronic book, a QR code scanner, free-form input with user-selectable interface elements and many others (Fig. 5).
Users also have the ability to create their own widgets and templates to use in their applications.
Once you have finished building the functionality, you can make the application available for download in accordance with your tariff plan. The free plan offers two ways to publish an app: creating a package for download on an Android device (which means we don't have to publish the app to the Google Play store) and creating an HTML5 app (which means we can test it on a device running iOS, without publishing the application in the AppStore and without purchasing an iOS developer license - Fig. 6).
In Fig. 7 shows screenshots of an iPhone 5 running a ready-made application downloaded from the link above.
Because we're using the free plan, the app includes an iBuildApp splash screen ad that appears when the app loads, an ad line on the title page, and banner ads that appear at the top of other pages when you open them (they can, however, be closed).
“Small Business” is not the only type of application available to users of the iBuildApp service. The range of potential consumers of ready-made applications and templates for this service is very diverse - from cafes and restaurants to religious organizations. For example, an e-book created from the appropriate template looks very good (Fig. 8).
In addition to applications for smartphones, the iBuildApp service also allows you to create applications for iPad. The choice of templates for this type of application is still small, and the finished application can only be tested in a browser (Fig. 9).
In addition to creating applications, the user of the service can create their own application templates (for this there is an online template editor that supports drag-and-drop operations) and sell them, as well as create their own widgets using XCode or Android SDK. But if you don’t want to deal with the issues of form design, purchasing a developer license and using development tools, you can get by with ready-made templates and widgets.
This concludes our acquaintance with the iBuildApp service for quickly generating mobile applications. It differs from similar services in the large number of templates and ready-made applications available for use, full support for the Russian language, not only in the applications created, but also in the online application editor, a very flexible pricing policy and a wide range of features available for free or for a nominal fee. Potential consumers of such a service can be companies and organizations of various profiles: cafes, restaurants, medical institutions, fitness clubs, retail enterprises, electronic stores, educational institutions, publishing houses, online stores, clubs. If such a consumer has a need for a Russian-language mobile application with more or less standard functionality, he should pay attention to this service - its use is highly likely to be much more effective than implementing or ordering individual development.
ComputerPress 12'2013
Operating principle
The basis of chemical current sources are two electrodes (a negatively charged anode containing a reducing agent and a positively charged cathode containing an oxidizing agent) in contact with the electrolyte. A potential difference is established between the electrodes - an electromotive force corresponding to the free energy of the redox reaction. The action of chemical current sources is based on the occurrence of spatially separated processes in a closed external circuit: at the negative anode, the reducing agent is oxidized, the resulting free electrons pass through the external circuit to the positive cathode, creating a discharge current, where they participate in the reduction reaction of the oxidizing agent. Thus, the flow of negatively charged electrons through the external circuit goes from the anode to the cathode, that is, from the negative electrode (the negative pole of the chemical current source) to the positive. This corresponds to the flow of electric current in the direction from the positive pole to the negative, since the direction of the current coincides with the direction of movement of positive charges in the conductor.
Modern chemical current sources use:
- as a reducing agent (anode material) - lead Pb, cadmium Cd, zinc Zn and other metals;
- as an oxidizing agent (cathode material) - lead(IV) oxide PbO2, nickel hydroxide NiOOH, manganese(IV) oxide MnO2 and others;
- as an electrolyte - solutions of alkalis, acids or salts.
general characteristics
Any electrochemical element is, in principle, a source of electric current. However, only a small part of these elements is suitable for practical use as a current source. This is due to the fact that the element must have a sufficiently large electrical capacity, high speed and turnover of electrochemical processes, stability during operation, manufacturability and cost-effectiveness of production. All chemical current sources (CHS) are divided into three groups: disposable current sources (galvanic cells), reusable current sources (batteries), fuel cells. In primary SDS, electrode materials are loaded into the cell during manufacture, and the cell is operated until its voltage drops to a certain critical value. Electrode materials of spent CDS go to waste or are partially recycled to regenerate components. In batteries, electroactive substances are produced during preliminary electrolysis (charging the battery). During operation, they are consumed (battery discharge), and the battery voltage drops to a certain maximum permissible value, after which they are charged again. The charging and discharging processes form the battery operating cycle. The maximum number of cycles (usually several hundred) depends on the type of battery and its operating conditions. The operation of the CDU is characterized by a number of parameters on which the possibility of using the CDU for certain needs depends. The electromotive force (EMF) of a chemical current source, like any electrochemical circle, is determined by the potential difference of the electrodes (anode and cathode) with the outer circle open. The total internal resistance r of a CDU is the resistance it exhibits when a direct current passes through it
where EF is the polarization emf: I is the current strength.
The first of these components, r0, is called ohmic resistance and is the sum of the resistances of the electrodes and electrolyte. The second component Rп is caused by a change in the potentials of the electrodes during the passage of current and is called polarization resistance, or fictitious resistance, its value depends on the magnitude of the current. During the discharge process of the CDS, the total internal resistance increases due to changes in the composition of the electrolyte and electrodes. The presence of internal resistance means that the discharge voltage Up (i.e., the voltage with a closed external circle) is always less than the emf of the current source:
(The subscript "p" means rank).
At a constant current and constant electrolyte temperature, the discharge voltage decreases over time. The charging voltage Uz of circulating systems is expressed by the equation:
At a constant value of the charging current, the charging voltage increases over time due to an increase in EF. At the end of the charge, when the process of electrolysis of water mainly occurs, the value of Uz stabilizes. Discharge capacity (current capacity) Qр also refers to the amount of electricity that can be obtained from the CDS under given operating conditions, i.e. at a given temperature, discharge current value and final value of discharge voltage.
RESULTS
RESULTS ²Ð½Ñ вÑÑодного напÑÑжениÑ, не завиÑÑÑего Ð¾Ñ Ð¿Ð¾ÑеблÑемого RESULTS smear ASSURANCE s ии и ÑегÑлиÑованиÑ.
ROOM знаÑением.
RESULTS CONTACT US › › › ROOM ROOM ROOM, OPTIONS ROOM ´Ð»Ñ ÑаР±Ð¾ÑнапÑÑжение.
Operating principle
The basis of chemical current sources are two electrodes (a positively charged anode containing a reducing agent and a negatively charged cathode containing an oxidizing agent) in contact with the electrolyte. A potential difference is established between the electrodes - an electromotive force corresponding to the free energy of the redox reaction. The action of chemical current sources is based on the occurrence of spatially separated processes in a closed external circuit: at the negative anode, the reducing agent is oxidized, the resulting free electrons pass through the external circuit to the positive cathode, creating a discharge current, where they participate in the reduction reaction of the oxidizing agent. Thus, the flow of negatively charged electrons through the external circuit goes from the anode to the cathode, that is, from the negative electrode (the negative pole of the chemical current source) to the positive. This corresponds to the flow of electric current in the direction from the positive pole to the negative, since the direction of the current coincides with the direction of movement of positive charges in the conductor.
Modern chemical current sources use:
- as a reducing agent (anode material) - lead Pb, cadmium Cd, zinc Zn and other metals;
- as an oxidizing agent (cathode material) - lead(IV) oxide PbO2, nickel hydroxide NiOOH, manganese(IV) oxide MnO2 and others;
- as an electrolyte - solutions of alkalis, acids or salts.
Fuel cells
Nowadays, most of the electricity is generated at thermal power plants by burning natural energy resources (coal, oil, natural gas). In this case, the process of converting the chemical energy of fuel into electrical energy goes through three stages: the conversion of chemical energy into thermal energy during fuel combustion, then thermal energy into mechanical work in a steam engine, and finally, the conversion of mechanical work into electricity in a generator. At all these stages, energy is lost and the efficiency factor (COP) of modern thermal power plants is about 40%, and for most power plants - 25%. Thermodynamic analysis carried out at the end of the 19th century. ., Showed that in galvanic cells there is no such limitation of efficiency as in heat engines. In 1893, Nernst calculated that if it were possible to convert the chemical energy of coal into electrical energy by electrochemical means, the maximum theoretical efficiency of such a process would be 99.75%. However, due to numerous technical difficulties, the first functional fuel cells were created only in the 20th century. . Fuel cells are galvanic cells in which the electrochemically active substances are ordinary combustible substances and oxygen, and the process of generating current is the oxidation of combustible substances. During operation of the element, there is a continuous supply of reagents and the appearance of reaction products, so that the composition of the system practically does not change. When any chemical current source operates, a total chemical reaction of interaction between an oxidizing agent and a reducing agent takes place. The maximum electrical work obtained by operating the current source is equal to the decrease in the isobaric potential for this reaction:
Converting energy into electricity using fuel cells is a rather complex process. The maximum electrical work obtained from a complex transformation is determined by the thermal effect of the reaction
The most reactive fuel is hydrogen. Hydrogen-oxygen cells are usually made using fine carbon or nickel electrodes immersed in an alkaline electrolyte solution. Schematically, such an element can be represented as follows:
(-) (Ni)H2 | KOH (30-40%) | O2 (Ni) (+)
Figure 4. Hydrogen-oxygen fuel cell
When the element operates on the negative electrode, an electrode reaction occurs:
On the positive
Total reaction
The theoretical value of the emf of a hydrogen-oxygen element at 250C is 1.229 V and does not depend on the composition of the electrolyte solution. When discharging hydrogen-oxygen elements, the voltage is kept within 07 - 0.9 V, depending on the discharge current density on the electrodes (in meadow element designs, the current density reaches 200-300 mA/cm2). Other types of gaseous fuels (carbon monoxide, hydrocarbons) can practically be used in fuel cells only at elevated temperatures (above C). In such high-temperature cells, either molten carbon salts of alkali metals or solid electrolytes with anionic (oxygen) conductivity are used as an electrolyte. Attempts to directly use solid coal in fuel cells have so far been unsuccessful. Coal can be used only after its preliminary gasification. If gasification is carried out using CO2, the following sequence of reactions is observed:
Gasification In fuel cell Total reaction
The hydrogen-oxygen element can be created, for example, using two platinum electrodes immersed in an aqueous solution of potassium hydroxide. One electrode is washed with hydrogen, the other with oxygen; Pt(H2) | KOH, saturated with H2 | KOH, saturated with O2 | (O2)Pt. In this element, the oxidation of hydrogen and the reduction of oxygen are spatially separated, and a current is generated during the reactions:
That is, the overall process comes down to the oxidation of hydrogen by oxygen with the formation of water. A significant disadvantage of such a fuel cell is its very low current density. To increase the current density, increased pressure and temperature, special electrode designs, solution mixing, etc. are used. The development of fuel cells continues. The possibility of using certain types of fuel in fuel cells and converting their chemical energy into electrical energy with a practical efficiency of up to % has been fundamentally proven.
Some types of chemical current sources
Galvanic cells
A galvanic cell is a chemical source of electric current, named after Luigi Galvani. The principle of operation of a galvanic cell is based on the interaction of two metals through an electrolyte, leading to the generation of electric current in a closed circuit.
| Type | Cathode | Electrolyte | Anode | Voltage, V |
| Manganese-zinc element | MnO2 | KOH | Zn | 1,56 |
| Manganese-tin element | MnO2 | KOH | Sn | 1,65 |
| Manganese-magnesium element | MnO2 | MgBr2 | Mg | 2,00 |
| Lead-zinc cell | PbO2 | H2SO4 | Zn | 2,55 |
| Lead-cadmium element | PbO2 | H2SO4 | Cd | 2,42 |
| Lead chlorine element | PbO2 | HClO4 | Pb | 1,92 |
| Mercury-zinc element | HgO | KOH | Zn | 1,36 |
| Mercury-cadmium element | HgO2 | KOH | Cd | 1,92 |
| Mercury-tin oxide element | HgO2 | KOH | Sn | 1,30 |
| Chrome-zinc element | K2Cr2O7 | H2SO4 | Zn | 1,8—1,9 |
Other types:
- Lead-fluorescent element
- Copper oxide galvanic cell
- Bismuth-magnesium element
- Mercury-bismuth-indium element
- Lithium chromium silver cell
- Lithium bismuthate element
- Lithium copper oxide cell
- Lithium-iodine-lead cell
- Lithium iodine cell
- Lithium thionyl chloride cell
- Lithium vanadium oxide cell
- Lithium fluoride cell
- Lithium bisulfur cell
- Dioxysulfate mercury element
- Magnesium sulfur element
- Lead chloride-magnesium element
- Silver-magnesium chloride element
- Copper-magnesium chloride element
- Zinc iodate element
- Magnesium perchlorate element
- Magnesium-m-DNB element
- Zinc-silver chloride element
- Chlorine-silver element
- Bromine-silver element
- Iodine-silver element
- Magnesium vanadium element
- Calcium chromate element
Electric batteries
An electric battery is a reusable chemical source of current (that is, unlike a galvanic cell, chemical reactions directly converted into electrical energy are many times reversible).
Electric batteries are used to store energy and autonomously power various devices. See also Category: Batteries.
- Iron-air battery
- Iron-nickel battery
- Lanthanum fluoride battery
- Lithium iron sulfide battery
- Li-ion battery
- Lithium polymer battery
- Lithium fluorine battery
- Lithium chlorine battery
- Lithium sulfur battery
- Manganese-tin element
- Sodium Nickel Chloride Battery
- Sodium-sulfur battery
- Nickel-cadmium battery
- Nickel metal hydride battery
- Nickel-zinc battery
- Lead-hydrogen battery
- Lead acid battery
- Lead-tin battery
- Silver cadmium battery
- Silver-zinc battery
- Zinc-bromine battery
- Zinc-air battery
- Zinc-chlorine battery
Fuel cells
A fuel cell is an electrochemical device similar to a galvanic cell, but differs from it in that the substances for the electrochemical reaction are supplied to it from outside - as opposed to the limited amount of energy stored in a galvanic cell or battery.
See also Category: Fuel cells.
- Direct methanol fuel cell.
- Solid oxide fuel cell.
- Alkaline fuel cell.
BATTERIES
BATTERIES electrical (from Latin accumulator - collector, storage device), chemical. multiple current sources. When charging from external source of electricity current in the battery accumulates energy, edges during discharge due to chemical. r-tion directly transforming. again into the electric one and is released into the external. chain. According to the principle of operation and basic The design elements of batteries do not differ from galvanic cells, but the electrode circuits, as well as the total current-generating circuit in batteries, are reversible. Therefore, after a battery has been discharged, it can be recharged by passing current in the opposite direction: positive. In this case, an oxidizing agent is formed on the electrode, and a reducing agent is formed on the negative electrode.
Naib. Lead-acid batteries are common, often called also acidic. Their action is based on the following:
Electrolyte - H2SO4 solution with a concentration of 12-24% by weight in a discharged state and 28-40% in a charged state. Open circuit voltage (OCV) is 1.95-2.15 V. Most often, electrodes are used from a paste containing a mixture of Pb3O4 and PbO with H2SO4 (active mass); this paste is spread on a profiled mesh-conductor made of Pb alloy with 2-12% Sb. The electrodes are formed by passing a charging current through the electrolyte solution in a certain mode; in this case, PbO2 is formed on one electrode, and Pb on the other. Then the electrodes are washed and dried under conditions that exclude the possibility of Pb oxidation. Batteries assembled from such electrodes, after filling the consumer with H2SO4 solution, are ready for use without recharging (other types of batteries require additional charge). Armored electrodes are also used, in which the active mass is enclosed in a perforated plastic or fabric tube.
The first lead battery was created by G. Plante in 1859. Now more than half of the world production of Pb is spent on the production of lead batteries with a unit capacity of 2-5000 A * h and beats. energy 25-40 W * h/kg. Basic advantages of such batteries: relates. low cost, flat discharge and charging curves, ability to work in different conditions. discharge modes; disadvantage - low service life (the number of permissible charge-discharge cycles for starter batteries is 100-300, for traction batteries with armored electrodes 800-1500). At the end of the charge, a noticeable release of gases is observed on the electrodes of a lead battery, which often carry along a mist of H2SO4 droplets. In this regard, much attention is paid to the creation of sealed lead batteries.
Alkaline nickel-cadmium (NKA) and nickel-iron (NIA) batteries are second in distribution after lead batteries. Current reaction:
where M-Cd or Fe. The electrolyte is an aqueous solution of KOH, into which LiOH is sometimes introduced to improve the performance of the nickel oxide electrode. NRC is 1.30-1.34 V for NCA and 1.37-1.41 V for NCA (after some time after the end of the charge), specification. energy 20-35 W*h/kg. Alkaline batteries, as a rule, have a high service life - 1-2 thousand cycles. Electrodes m.b. diff. designs: lamella, in which the active mass is enclosed in flat boxes-lamellas made of perforated steel tape; sintered, in which the active mass is introduced into the pores of the base formed by sintering powdered metal. Ni; pressed, in which the active mass is under pressure. 35-60 MPa is pressed onto a steel base (plate thickness 0.8-1.8 mm).
NJA is mainly used. for the production of high-capacity traction batteries (up to 1200 A * h). They are cheaper than NKA, but have higher characteristics. self-discharge due to corrosion of iron in an alkaline solution; in addition, they have lower current and energy output values. In the NKA there is no Cd corrosion and associated gas evolution, which makes it possible to maintain the charged state for a longer period of time and the possibility of completely sealing the battery. Sealed NKVs are produced with capacities from 0.01 to 160 A * h. They are widely used as sources of electricity. energy in household appliances, communications equipment, etc.
Silver-zinc batteries with alkaline electrolyte have a high specification. energy (up to 130 Wh/kg) and can be discharged with high currents, but due to the high cost of silver they are used only in special industries, for example. in space technology. Current-generating circuit:
During charging, the formation of AgO is also possible. Therefore, steps corresponding to reactions involving Ag2O and AgO are observed on the charge and discharge curves. NRC 1.60-1.85 V, resource does not exceed 100-200 cycles.
Attempts to replace Ag with other materials led to the creation of nickel-zinc batteries, in which a sintered or pressed nickel oxide electrode from NKA and a zinc electrode from silver-zinc batteries are used. Current-generating circuit:
NRC 1.74-1.78 V, beat. energy approx. 60 Wh/kg, resource approx. 300 cycles. The developed versions of these batteries are designed mainly. for electric vehicles, but their widespread use is hampered by their insufficient service life.
Nickel-hydrogen batteries have a trace of leakage. current-generating circuit:
The H2 released during charging accumulates under pressure. Therefore, the block with electrodes is placed in a steel cylinder that can withstand pressures of up to 10 MPa. NRC 1.32-1.36 V, beat. energy 50-60 W*h/kg, resource several. thousand cycles. Due to the high cost of production, such batteries are currently used only in space applications. technology.
Among the promising designs of batteries with non-aqueous electrolytes is Naib. Of interest are sodium sulfur with hard ceramic. electrolyte made of sodium aluminates, which conducts through Na+ ions. The operating temperature of such a battery is 300-350°C. Current-generating circuit:
NRC 2.08 V. Main. development difficulty: creating a technology for manufacturing thin but sufficiently durable parts from solid electrolyte. High-temperature sulfide-iron-lithium batteries are also being developed; in them, instead of a solid electrolyte, molten salts are used, FeS or FeS2 are used as oxidizing agents. In terms of their characteristics, these batteries are close to sodium sulfur batteries.
If a higher voltage is required than that of a single battery, batteries are used, consisting of batteries connected in series with a common housing, terminals and markings. Batteries are widely used in transport systems for starting engines, lighting, etc. Traction batteries are used for power plants of electric cars, high-capacity stationary batteries are used to power telephone networks, as emergency sources of electricity in case of power outages (for example, in operating rooms). Small-sized sealed batteries are used to power portable radios and other devices. Much attention is paid to the development of batteries for electric vehicles. The global production of starter batteries from lead-acid batteries alone exceeds 100 million units per year.
Unlike galvanic battery elements require care during operation: they must be charged, periodically top up with electrolyte and maintain its concentration constant, carry out training and control charge-discharge cycles, etc. They are developing the so-called. low-maintenance and maintenance-free batteries, the maintenance of which is simplified.
=== Use. literature for the article “BATTERIES” : Romanov V.V., Khashev Yu.M., Chemical current sources, 2nd ed., M., 1978; Bagotsky V.S., Skundin A.M., Chemical current sources, M., 1981. BC Bagotsky.
“BATTERIES” page was prepared based on materials from the chemical encyclopedia.
Some types of chemical current sources
Galvanic cells
A galvanic cell is a chemical source of electric current, named after Luigi Galvani. The principle of operation of a galvanic cell is based on the interaction of two metals through an electrolyte, leading to the generation of electric current in a closed circuit.
| Type | Cathode | Electrolyte | Anode | Voltage, V |
| Manganese-zinc element | MnO2 | KOH | Zn | 1,56 |
| Manganese-tin element | MnO2 | KOH | Sn | 1,65 |
| Manganese-magnesium element | MnO2 | MgBr2 | Mg | 2,00 |
| Lead-zinc cell | PbO2 | H2SO4 | Zn | 2,55 |
| Lead-cadmium element | PbO2 | H2SO4 | Cd | 2,42 |
| Lead chlorine element | PbO2 | HClO4 | Pb | 1,92 |
| Mercury-zinc element | HgO | KOH | Zn | 1,36 |
| Mercury-cadmium element | HgO2 | KOH | Cd | 1,92 |
| Mercury-tin oxide element | HgO2 | KOH | Sn | 1,30 |
| Chrome-zinc element | K2Cr2O7 | H2SO4 | Zn | 1,8—1,9 |
Other types:
- Lead-fluorescent element
- Copper oxide galvanic cell
- Bismuth-magnesium element
- Mercury-bismuth-indium element
- Lithium chromium silver cell
- Lithium bismuthate element
- Lithium copper oxide cell
- Lithium-iodine-lead cell
- Lithium iodine cell
- Lithium thionyl chloride cell
- Lithium vanadium oxide cell
- Lithium fluoride cell
- Lithium bisulfur cell
- Dioxysulfate mercury element
- Magnesium sulfur element
- Lead chloride-magnesium element
- Silver-magnesium chloride element
- Copper-magnesium chloride element
- Zinc iodate element
- Magnesium perchlorate element
- Magnesium-m-DNB element
- Zinc-silver chloride element
- Chlorine-silver element
- Bromine-silver element
- Iodine-silver element
- Magnesium vanadium element
- Calcium chromate element
Electric batteries
An electric battery is a reusable chemical source of current (that is, unlike a galvanic cell, chemical reactions directly converted into electrical energy are many times reversible).
Electric batteries are used to store energy and autonomously power various devices. See also Category: Batteries.
- Iron-air battery
- Iron-nickel battery
- Lanthanum fluoride battery
- Lithium iron sulfide battery
- Li-ion battery
- Lithium polymer battery
- Lithium fluorine battery
- Lithium chlorine battery
- Lithium sulfur battery
- Manganese-tin element
- Sodium Nickel Chloride Battery
- Sodium-sulfur battery
- Nickel-cadmium battery
- Nickel metal hydride battery
- Nickel-zinc battery
- Lead-hydrogen battery
- Lead acid battery
- Lead-tin battery
- Silver cadmium battery
- Silver-zinc battery
- Zinc-bromine battery
- Zinc-air battery
- Zinc-chlorine battery
Fuel cells
A fuel cell is an electrochemical device similar to a galvanic cell, but differs from it in that the substances for the electrochemical reaction are supplied to it from outside - as opposed to the limited amount of energy stored in a galvanic cell or battery.
See also Category: Fuel cells.
- Direct methanol fuel cell.
- Solid oxide fuel cell.
- Alkaline fuel cell.
Classification
The most common types are galvanic cells and batteries. Almost everyone is familiar with them. But the classification of such devices is broader and also assumes the existence of fuel cells.
Current source classification scheme
Galvanic cells
The galvanic cell got its name in honor of the scientist Galvano, who discovered the wonderful possibility of producing electric current by creating a simple structure of electrolyte and electrodes. They are considered the first prototypes of modern devices for generating electricity through chemical reactions.
You might be interested in: How does electric current affect the human body?
Chemical current sources - galvanic cells and batteries
Note! Currently, this device is more compact and safer to use; it is a regular battery. The peculiarity of the operation of such a device is that it is one-time use. After the final decomposition of the electrolyte into substances, it is impossible to recharge them for the following reactions.
Electric batteries
An electric battery is a more versatile version of the device that can be charged several times after losing its electrolyte charge. This feature is explained by the regeneration of substances that form the electrolyte.
Battery device
In this case, charging is carried out from an external (external) current source. Motorists often encounter this need to restore the reagent in batteries when charging the battery.
Fuel cells
An electrochemical fuel cell is a promising source that is quite important for creating comfortable and, in some situations, vital living conditions.
Thermal chemical source
The peculiarity of the operation of such an element is as follows. Each time, a certain portion of electrolyte is supplied to the electrodes, which, after discharge, is removed from the structure. For example, thanks to this operating principle, a backup current generator can produce electricity for 10-15 years.
Note! After the expiration date, operation can be extended if power is restored.
ROOM
RESULTS › › › › RESULTS ¾Ð²ÑÑиÑÑоÑников поÑÑоÑнного Ñока.
ROOM RESULTS °ÑаÑеР¸. RESEARCH, RESULTS µ µ RESULTS RESULTS RESULTS 15%.