18K 2 7 min.
In 2010, Konstantin Novoselov and Andrei Geim were awarded the Nobel Prize for pioneering experiments in the study of a two-dimensional material - graphene. Graphene is a two-dimensional structure in which carbon atoms are arranged at the vertices of regular hexagons. Graphene is the constituent unit of graphite and is used as a theoretical model to describe other allotropic forms of carbon such as fullerenes and nanotubes. Although the first laboratory experimental samples of graphene were obtained relatively recently, there is already a lot of research on the use of graphene in various fields. Kommersant-Science talks about some of them.
Photo: Courtesy of Graphenox
Photo: Courtesy of Graphenox
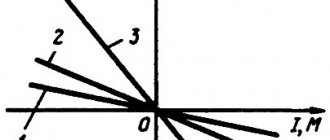
Graphene has unique electronic and optical properties related to its band structure. In the first Brillouin zone of graphene, there are special points K and K`, near which the electron energy depends linearly on the wave vector. Thus, graphene is a semiconductor with a zero band gap, and the movement of electrons in it is described not by the Schrödinger equation, as in bulk semiconductors, but by the Dirac equation for massless quasiparticles. As a result, graphene exhibits a half-integer quantum Hall effect and ultra-high electron mobility. Graphene also has outstanding optical characteristics. For example, the amount of optical absorption of light in it is 2.3% of the intensity of the incident radiation and does not depend on the wavelength.
In the last two years, a breakthrough has been made in understanding the properties of disordered graphene. For example, in 2022, a fundamental discovery was made - superconductivity in twisted graphene. American physicists have proposed a model that qualitatively explains the phenomenon of superconductivity.
Interview with Nobel laureate Konstantin Novoselov
Alexey Arsenin, Deputy Director of the Center for Photonics and Two-Dimensional Materials at MIPT, drew our attention to work with hybrid structures that combine two or more two-dimensional materials, including layers of two-dimensional materials rotated relative to each other. If we make a cavity in the material one layer thick, then this is another object for research - 2D nothing. In 2019, a doctoral dissertation on graphene was defended in Chernogolovka by Pavel Ostrovsky from the Institute of Theoretical Physics. L. D. Landau. In particular, he constructed a complete symmetry classification of possible types of impurities in graphene. Professor Oleg Yazev from EPFL (Ecole Polytechnique Federale de Lausanne, Switzerland) described Ostrovsky’s work to us as follows: “The dissertation combines a series of theoretical works aimed at understanding electronic conductivity in graphene, taking into account its two-dimensionality and unique electronic structure, properties that make it so different from known metals and conductors. Particular attention is paid to electronic conductivity in the presence of impurities.”
Defects
The apparent ease of producing graphene is inextricably linked with a fundamental problem - the thermodynamic stability of two-dimensional conductors. The new nanomaterial, represented by layered crystals, belongs to 2D systems. Two-dimensional layered structures, having metallic properties, are thermodynamically extremely unstable.
When the ambient temperature drops, graphene materials lose the properties of metals. That is, a transition occurs from metal to dielectric. The problem requires further research.
Applications of Graphene
Nobel laureate Konstantin Novoselov (Manchester) in an exclusive interview for Kommersant-Science said that it is difficult for him to estimate the global production of graphene in tons or money. As for the largest consumers of graphene, he noted the following (not all!):
- Huawei uses graphene for thermoregulation of smartphones;
- BYD (China) uses graphene in batteries;
- Samsung is planning (or already using) graphene in silicon chips to control contact resistance;
- in the automotive industry, a number of companies, for example Ford Motor, use polymer composites with graphene filler;
- Canadian Ora Graphene Audio Inc. produces composite material for wireless acoustic headphones GRAPHENEQ TM.
How to improve the characteristics of existing electronic storage devices to perfection?
If graphene is added to conventional materials for the manufacture of battery electrodes, their characteristics will be significantly improved. Batteries made using graphene technology can delight consumers with low weight, durability, and impressive capacity. One cannot help but admire the speed of charging of such equipment.
Graphene has the ability to improve the properties of an electronic storage device using different methods. Specifically, we are talking about energy density and shape. The result is that lithium-ion electric batteries, and other types of batteries, can be improved by introducing graphene into the anode of the electric battery. Also, scientists have found that the development of hybrid materials may be important for improving the quality of the storage device. For example, the symbiosis of graphene and vanadium oxide catalysis can be used on Li-ion cathodes. It is able to provide an accelerated charging process, as well as high stability of the charging cycle. In this situation, the hybrid will have increased energy intensity and low electrical conductivity, and it is graphene that serves as the basis for all this.
Scientists are spending considerable resources on finding new types of active material for electrodes, as this will help bring batteries to a completely new level of productivity and survivability. In addition, they want to better adapt such equipment for larger devices. Nanostructured materials for lithium-ion batteries may be a good solution.
For example, workers at the University of Vienna, in collaboration with international scientists, have created a new nanostructured anode substance for lithium-ion batteries, which increases the service life and capacity of electronic storage devices.
A 2D/3D nanocomposite based on mixed metal oxide and graphene, invented by a pair of scientists and their colleagues, can significantly improve the electrochemical performance of Li-ion batteries. This material is quite capable of organizing a new approach to the more rational use of storage devices in large devices - these are electric or hybrid vehicles. Naturally, a more advanced material, as always, outperforms the lithium-ion design: batteries based on it stably maintain their functionality for 3000 charge/discharge cycles, but Li-ion electric storage devices lose their effective performance after about 1000 charge cycles.
Interview with Professor, Doctor of Chemical Sciences Daria Andreeva
“Right now, one of the most popular applications of graphene is as a heat sink in electronic devices. Our laboratory currently has a project aimed at solving this problem. We are developing a thermally conductive paste for microelectronics based on our graphene nanoplates,” Maxim Rybin, General Director of Rusgrafen LLC (Protvino), told us. In addition, Rusgrafen LLC, together with GraphenOx LLC (Chernogolovka), learned how to make various types of graphene paints and inks for flexible electronics. “We can apply a thin layer of ink as the active element of the sensor and electrically conductive paints as electrodes,” explained Maxim Rybin.
According to Galina Chernik, General Director of Aktiv-nano LLC (St. Petersburg), the company has developed finely split graphite (few-layer graphene, few-layer graphene). The product is manufactured using mechanical methods, without chemical reagents and high temperatures. There is no oxidation of the carbon material. The specific surface area of few-layer graphene is 250–500 square meters. m/g, which corresponds to an average thickness of five to ten layers of carbon atoms. The specific electrical conductivity of the material reaches 100–200 siemens per centimeter, which is several times higher than that of electrically conductive carbon black. Few-layer graphene powder can be used in electrically conductive and thermally conductive materials and as a solid lubricant in powder metallurgy.
Obtaining graphene using the adhesive tape method
To repeat the experiment of the Nobel laureates, you will need adhesive tape and a piece of graphite. The tape needs to be glued to the graphite. When you peel the film away from the material, there will be a layer of substance left on it. But it will still not be thin enough to be called graphene and gain unique properties. This layer must be removed several more times with another tape of tape until a layer of substance 1 atom thick remains, which can only be seen through a microscope.
Position of the Russian government
Watch
In 2022, a group of projects, Graphene Technology Group, was formed, co-founded by Maxim Gudkov, Maxim and Maxim Rabchinsky. The group includes projects Graphene Technology, GraphSensors and GraphApta (Graph-SK LLC, GraphSensors LLC). The group has developed a low-cost and scalable method for the synthesis of graphene oxide, which makes it possible to obtain high-purity graphene oxide (impurity fraction does not exceed 0.1 at.%) with the required monolayer particle sizes in the range from 500 nm to 100 μm. Scalable methods have also been developed for preparing a set of functionalized graphenes with a controlled composition of functional groups. According to project manager Maxim Gudkov, the materials produced are of great interest for the electronics industry (touch screens, supercapacitors, various sensors and microelectronic chips), the composite materials industry (aerospace industry, medical implants, engineering materials), for the catalytic industry (carriers for hydrogen energy catalysts) and others. Over the coming year, the company plans to increase production volume to 10 kg of graphene oxide per month, which will reduce the price from the current 695 rubles. for 1 g up to 280 rub.
Receipt at home
Is it possible to make graphene at home? It turns out yes! You just need to take a kitchen blender with a power of at least 400 W and follow the method developed by Irish physicists.
How to make graphene at home? To do this, pour 500 ml of water into the blender bowl, adding 10-25 milliliters of any detergent and 20-50 grams of crushed lead to the liquid. Next, the device should operate for 10 minutes to half an hour, until a suspension of graphene flakes appears. The resulting material will have high conductivity, which will allow it to be used in photocell electrodes. Also, graphene produced at home can improve the properties of plastic.
Graphene market in the USA
The GraphSensors project is aimed at the development and production of highly selective multisensor gas chips based on graphene, which have a number of unique characteristics: a large set of diagnosed and identified gases (the sensor is capable of recognizing up to seven gases in a mixture), low power consumption (5–10 mW), the ability to operate in an oxygen-free environment atmosphere and no need to heat the chip. Project manager Maxim Rabchinsky commented: “Our chips can be used both for diagnosing the content of components in gas mixtures and for more complex tasks: diagnosing human diseases (Breath Biopsy), identifying various odors, such as coffee, tobacco, wine, meat, a list of practically is not limited. The chips can be integrated both into “Electronic nose” systems and into classic multi-channel gas analyzers.”
The GraphApta project is aimed at developing portable test systems for personal use for regular diagnosis of the course of infectious diseases, such as HIV, hepatitis, and sexually transmitted diseases. The test systems are represented by disposable test strips based on graphene coatings and a compact measuring unit, a hepatometer, with access to a cloud service for storing data on the dynamics of the disease. Using the system will allow you to carry out diagnostics at any time and anywhere, by analogy with a conventional glucometer, with high selectivity, sensitivity, as well as simplicity and low cost of the device. According to project manager Ivan Komarov, it is planned to create both personal devices and commercial solutions with advanced performance characteristics for companies involved in health monitoring and analysis of viral diseases.
Automotive use
According to researchers, the specific energy intensity of graphene is close to 65 kWh/kg. This figure is 47 times higher than that of the now so common lithium-ion batteries. Scientists used this fact to create a new generation of chargers.
A graphene-polymer battery is a device that stores electrical energy as efficiently as possible. Currently, work on it is being carried out by researchers from many countries. Spanish scientists have achieved significant success in this matter. The graphene-polymer battery they created has an energy capacity hundreds of times higher than that of existing batteries. It is used to equip electric vehicles. A car with a graphene battery can travel thousands of kilometers without stopping. It will take no more than 8 minutes to recharge an electric vehicle when the energy resource is exhausted.
James Baker, Director, Graphene Engineering Innovation Center
— Mr. Baker, what technologies are used in the European Union for the production of graphene?
— There are two main methods (but there are many variations) for producing graphene. The top-down technique starts with graphite, which is split through various methods into layers or plates of graphene. Bottom-up technique starts with carbon atoms or carbon-containing gas, such as CH4 (methane), and through processes such as CVD (chemical vapor deposition), a film of graphene is formed on a substrate sheet (such as copper ).
Read
Look
NIIgrafit JSC (Moscow) has developed flexible piezoelectric sensors with graphene contacts, graphene-containing highly anisotropic heat-dissipating plastics with a thermal conductivity coefficient of over 200 W/mK in one direction and 10–20 W/mK in the other, with heat resistance up to 180°C and strength over 50 MPa. The ongoing projects are focused on the development of a competitive technological process for producing graphene from natural graphite using liquid-phase exfoliation. The result should be a commercial line of suspensions of graphene preparations that can be used to modify polymers and composite materials, to obtain liquid coolants, lubricants, conductive contacts, inks, and optical coatings.
Graphene: myths and reality
From the editor: touching on the topic of modernization of the Russian economy and the development of high technologies in our country, we set the task not only to draw the attention of readers to the shortcomings, but also to tell about positive examples. Moreover, there are such, and quite a few. Last week we talked about the development of fuel cells in Russia, and today we’ll talk about graphene, for studying the properties of which “our former people” recently received a Nobel Prize. It turns out that in Russia, or more precisely in Novosibirsk, they are working on this material very seriously.
Silicon, as the basis of microelectronics, has firmly gained a position in the high-tech space, and this did not happen by chance. Firstly, it is relatively easy to impart the desired properties to silicon. Secondly, it has been known to science for a long time, and has been studied far and wide. The third reason is that truly enormous amounts of money have been invested in silicon technology, and few people will dare to bet on the new material now. After all, this will require rebuilding a huge industrial sector. Or rather, build it almost from scratch.
However, there are other contenders for leadership as a semiconductor material. For example, graphene, which became very fashionable after the Nobel Prize was awarded for studying its properties. There are indeed reasons to switch to it from silicon, since graphene has a number of significant advantages. But whether we will eventually get “electronics on graphene” is not yet clear, because along with the advantages there are also disadvantages.
To talk about the prospects of graphene in microelectronics and its unique properties, we met in Novosibirsk with the chief researcher of the Institute of Inorganic Chemistry named after. A.V. Nikolaev SB RAS, Doctor of Chemical Sciences, Professor Vladimir Fedorov.
Alla Arshinova: Vladimir Efimovich, what is the current position of silicon in microelectronics?
Vladimir Fedorov: Silicon has been used in the industry for a very long time as the main semiconductor material. The fact is that it is easily doped, that is, atoms of various elements can be added to it, which specifically change the physical and chemical properties. This modification of high-purity silicon makes it possible to obtain n- or p-type semiconductor materials. Thus, directional doping of silicon regulates the functional properties of materials that are important for microelectronics.
Silicon is truly a unique material, and this is the reason why so much effort, money and intellectual resources have been invested in it. The fundamental properties of silicon have been studied in such detail that there is a widespread belief that there simply cannot be a replacement for it. However, recent research into graphene has given the green light to another view, which is that new materials could be developed to the point where they could replace silicon.
Crystal structure of silicon
Such discussions arise periodically in science, and, as a rule, they are resolved only after serious research. For example, recently there was a similar situation with high-temperature superconductors. In 1986, Bednorz and Müller discovered superconductivity in barium-lanthanum-copper oxide (for this discovery they were awarded the Nobel Prize in 1987 - a year after the discovery!), which was detected at temperatures significantly higher than the values characteristic of previously known time of superconducting materials. Moreover, the structure of cuprate superconducting compounds differed significantly from low-temperature superconductors. Then an avalanche of studies of related systems led to the production of materials with a superconducting transition temperature of 90 K and higher. This meant that instead of expensive and capricious liquid helium, liquid nitrogen could be used as a coolant - there is a lot of it in gaseous form in nature, and besides, it is significantly cheaper than helium.
But, unfortunately, this euphoria soon faded after careful research into new high-temperature superconductors. These polycrystalline materials, like other complex oxides, are like ceramics: they are brittle and non-ductile. It turned out that within each crystal superconductivity has good parameters, but in compact samples the critical currents are quite low, which is due to weak contacts between the grains of the material. Weak Josephson junctions between superconducting grains do not make it possible to produce a material (for example, make a wire) with high superconducting characteristics.
Solar battery based on polycrystalline silicon
The same situation can happen with graphene. At present, very interesting properties have been found for it, but extensive research remains to be done to definitively answer the question of the possibility of producing this material on an industrial scale and using it in nanoelectronics.
Alla Arshinova: Please explain what graphene is and how it differs from graphite?
Vladimir Fedorov: Graphene is a monoatomic layer formed from carbon atoms, which, like graphite, has a honeycomb-shaped lattice. And graphite is, accordingly, graphene layers stacked on top of each other. The layers of graphene in graphite are connected to each other by very weak van der Waals bonds, which is why it is ultimately possible to tear them apart from each other. When we write with a pencil, this is an example of us removing layers of graphite. True, the trace of a pencil remaining on paper is not yet graphene, but a graphene multilayer structure.
Now every child can seriously claim that he is not just transferring paper, but creating a complex graphene multilayer structure
But if it is possible to split such a structure down to one layer, then true graphene is obtained. Similar splittings were carried out by this year’s Nobel laureates in physics, Geim and Novoselov. They managed to split graphite using tape, and after studying the properties of this “graphite layer”, it turned out that it has very good parameters for use in microelectronics. One of the remarkable properties of graphene is its high electron mobility. They say that graphene will become an indispensable material for computers, phones and other equipment. Why? Because in this area there is a tendency to speed up information processing procedures. These procedures are related to clock speed. The higher the operating frequency, the more operations can be processed per unit time. Therefore, the speed of charge carriers is very important. It turned out that charge carriers in graphene behave like relativistic particles with zero effective mass. These properties of graphene really give hope that it will be possible to create devices capable of operating at terahertz frequencies, which are inaccessible to silicon. This is one of the most interesting properties of the material.
Nobel laureates in physics 2010 Andre Geim and Konstantin Novoselov
Flexible and transparent films can be obtained from graphene, which is also very interesting for a number of applications. Another plus is that it is a very simple and very light material, lighter than silicon; Besides, there is plenty of carbon in nature. Therefore, if they really find a way to use this material in high technologies, then, of course, it will have good prospects and, perhaps, will eventually replace silicon.
But there is one fundamental problem associated with the thermodynamic stability of low-dimensional conductors. As is known, solids are divided into various spatial systems; for example, the 3D (three-dimensional) system includes volumetric crystals. Two-dimensional (2D) systems are represented by layered crystals. And chain structures belong to a one-dimensional (1D) system. So, low-dimensional - 1D chain and 2D layered structures with metallic properties are not stable from a thermodynamic point of view; as the temperature decreases, they tend to turn into a system that loses its metallic properties. These are the so-called metal-dielectric transitions. How stable graphene materials will be in some devices remains to be seen. Of course, graphene is interesting, both from the point of view of electrophysical properties and mechanical ones. The monolithic layer of graphene is believed to be very strong.
Alla Arshinova: Stronger than diamond?
Vladimir Fedorov: Diamond has three-dimensional bonds and is mechanically very strong. In graphite, the interatomic bonds in the plane are the same, perhaps even stronger. The fact is that from a thermodynamic point of view, diamond should turn into graphite, because graphite is more stable than diamond. But in chemistry there are two important factors that control the transformation process: the thermodynamic stability of the phases and the kinetics of the process, that is, the rate of transformation of one phase into another. So, diamonds have been lying in museums around the world for centuries and do not want to turn into graphite, although they should. Maybe in millions of years they will still turn into graphite, although it would be a great pity. The process of diamond turning into graphite at room temperature occurs at a very slow rate, but if you heat the diamond to a high temperature, then the kinetic barrier will be easier to overcome, and this will definitely happen.
Graphite in its original form
Alla Arshinova: The fact that graphite can be split into very thin flakes has been known for a long time. What then was the achievement of the 2010 Nobel laureates in physics?
Vladimir Fedorov: You probably know such a character as Petrik. After presenting the Nobel Prize to Andrei Geim and Konstantin Novoselov, he stated that the Nobel Prize had been stolen from him. In response, Geim said that, indeed, such materials had been known for a very long time, but they were given the prize for studying the properties of graphene, and not for discovering a method for its production as such. In fact, their merit is that they were able to split off very good quality graphene layers from highly oriented graphite and study their properties in detail. The quality of graphene is very important, as in silicon technology. When they learned how to obtain silicon of a very high degree of purity, only then did electronics based on it become possible. The situation is the same with graphene. Geim and Novoselov took very pure graphite with perfect layers, managed to split off one layer and studied its properties. They were the first to prove that this material has a set of unique properties.
Alla Arshinova: In connection with the awarding of the Nobel Prize to scientists with Russian roots working abroad, our compatriots who are far from science are wondering whether it was possible to achieve the same results here in Russia?
Vladimir Fedorov: Probably it was possible. They just left at the right time. Their first article, published in Nature, was co-authored with several scientists from Chernogolovka. Apparently, our Russian researchers also worked in this direction. But it was not possible to complete it in a convincing manner. It's a pity. Perhaps one of the reasons is more favorable conditions for working in foreign scientific laboratories. I recently came from Korea and can compare the working conditions I was given there with working at home. So, there I was not preoccupied with anything, but at home there were a lot of routine duties that took up a lot of time and constantly distracted me from the main thing. I was provided with everything I needed, and this was done with amazing speed. For example, if I need some kind of reagent, I write a note and they bring it to me the next day. I suspect that Nobel laureates also have very good working conditions. Well, they had enough persistence: they tried many times to get good material and finally achieved success. They really spent a lot of time and effort on this, and in this sense the award was deservedly awarded.
Alla Arshinova: What advantages does graphene provide compared to silicon?
Vladimir Fedorov: Firstly, we have already said that it has high carrier mobility; as physicists say, charge carriers do not have mass. Mass always slows down movement. And in graphene, electrons move in such a way that they can be considered massless. This property is unique: if there are other materials and particles with similar properties, they are extremely rare. This is what graphene turned out to be good for, and this is also why it compares favorably with silicon.
Secondly, graphene has high thermal conductivity, and this is very important for electronic devices. It is very light and the graphene sheet is transparent and flexible and can be rolled up. Graphene can be very cheap if optimal methods for its production are developed. After all, the “scotch tape method” demonstrated by Game and Novoselov is not industrial. This method produces samples of really high quality, but in very small quantities, only for research.
And now chemists are developing other ways to produce graphene. After all, you need to obtain large sheets to put graphene production on stream. We are also dealing with these issues here at the Institute of Inorganic Chemistry. If they learn to synthesize graphene using methods that would make it possible to produce high-quality material on an industrial scale, then there is hope that it will revolutionize microelectronics.
Alla Arshinova: As everyone probably already knows from the media, a graphene multilayer structure can be obtained using a pencil and adhesive tape. What is the technology for producing graphene used in scientific laboratories?
Vladimir Fedorov: There are several methods. One of them has been known for a very long time; it is based on the use of graphite oxide. Its principle is quite simple. Graphite is placed in a solution of highly oxidizing substances (for example, sulfuric, nitric acid, etc.), and when heated, it begins to interact with oxidizing agents. In this case, the graphite is split into several sheets or even monatomic layers. But the resulting monolayers are not graphene, but are oxidized graphene, which contains attached oxygen, hydroxyl and carboxyl groups. Now the main task is to restore these layers to graphene. Since oxidation produces small particles, they must be glued together in some way to obtain a monolith. The efforts of chemists are aimed at understanding how it is possible to make a graphene sheet from graphite oxide, the production technology of which is known.
There is another method, also quite traditional and known for a long time - this is chemical vapor deposition with the participation of gaseous compounds. Its essence is as follows. First, the reaction substances are sublimated into the gas phase, then they are passed through a substrate heated to high temperatures, on which the desired layers are deposited. Once a starting reagent, such as methane, is selected, it can be decomposed in such a way that the hydrogen is split off and the carbon remains on the substrate. But these processes are difficult to control, and it is difficult to obtain an ideal layer.
Graphene is one of the allotropic modifications of carbon
There is another method that is now beginning to be actively used - the method of using intercalated compounds. In graphite, as in other layered compounds, molecules of various substances, called “guest molecules,” can be placed between the layers. Graphite is the matrix of the “host”, where we supply the “guests”. When guests intercalate into the host's lattice, the layers naturally separate. This is exactly what is required: the intercalation process breaks down the graphite. Intercalated compounds are very good precursors for producing graphene - you just need to remove the “guests” from there and prevent the layers from collapsing back into graphite. An important step in this technology is the process of obtaining colloidal dispersions that can be converted into graphene materials. At our institute we support exactly this approach. In our opinion, this is the most advanced direction, from which very good results are expected, because isolated layers can be obtained most simply and efficiently from various types of intercalated compounds.
Graphene's structure is similar to a honeycomb. And recently it has become a very “sweet” topic
There is another method, which is called total chemical synthesis. It lies in the fact that the necessary “honeycombs” are assembled from simple organic molecules. Organic chemistry has a very developed synthetic apparatus, which allows one to obtain a huge variety of molecules. Therefore, they are trying to obtain graphene structures by chemical synthesis. So far, it has been possible to create a graphene sheet consisting of about two hundred carbon atoms.
Other approaches to graphene synthesis are being developed. Despite numerous problems, science in this direction is successfully moving forward. There is a high degree of confidence that existing obstacles will be overcome, and graphene will bring a new milestone in the development of high technologies.
Import of graphene products
In 2022, the promotion of imported graphene products to the Russian market began. Thus, Alfarok Materials LLC (Moscow) imported Graphenestone photocatalytic paint from Spain. Andrey Buslaev, executive director of GC Genesis GNP LLC (Moscow), told Kommersant-Nauka about the launch of the Swiss motor oil Genesis GNP Engine Oil and transmission oil Genesis GNP Gear Oil in the Russian and CIS markets in August of this year. with graphene additives.
Tests have shown that the coefficient of friction in the new engine oil is reduced from 0.12 to 0.02, the engine wear rate is reduced by two to three times, and vehicle fuel consumption drops by 30% on naturally aspirated engines and by 15% for turbo engines.
New material
Nanotechnology has allowed scientists to make a carbon plate harder than diamond that is only one atom thick. It consists of graphene. This is the thinnest and strongest material in the entire Universe, which transmits electricity much better than silicon in computer chips.
The discovery of graphene is considered a real revolutionary event that will change a lot in our lives. This material has such unique physical properties that it radically changes a person’s understanding of the nature of things and substances.
Liu Zhongfan, Beijing Graphene Institute
— Please indicate the leading graphene production companies in China.
— According to the Global Graphene Index released by the China Economic Information Service (CEIS) in 2022, China and the United States are leading the graphene industry. In general, graphene production can be classified into CVD methods for producing graphene film and methods for producing graphene powders. Some examples of Chinese graphene manufacturers: CVD films (Beijing Graphene Institute, Chongqing Graphene Technology Co., Ltd., 2D Carbon (Changzhou) Tech Inc., Ltd., Wuxi Graphene film Co., Ltd., Nanjing Ji Cang Nano Tech Co ., Ltd.); graphene powders (SuperC (Dongguan) New Materials Technology Co., Ltd., Ningbo Morsh Co., Ltd., Qingdao Haoxin Technology Co., Ltd., Xiamen Knano Graphene Technology Co., Ltd., Baotailong Co. Ltd., The Sixth Elements (Changzhou) Materials Technology Co., Ltd., Shandong Leadernano Technology Co., Ltd.).
Read
Look
Financial difficulties during project implementation
Today there is such a problem as a huge number of companies engaged in research in the field of power supplies. New developments appear like mushrooms after rain, but there is no obvious favorite among all this fraternity. This state of affairs also affects investors, who are clearly in no hurry to part with their capital when investing money in new ventures.
By the way, you need quite a lot of money: to organize a small production line for the production of high-tech batteries, you will need something like 500 million dollars. In addition, creating a promising source of energy is only half the battle; the scientific project still needs to be put on a “commercial track,” which is not an easy maneuver.
Creators of mobile devices or manufacturers of electric cars will test new equipment for years before approving a profitable option. Investments during this period will not pay off, and the development company will operate at a loss. Do you think that if a new technology hits the market, that’s all, success, luck, the job is done and the game is played? No matter how it is, it turns out that this is still not enough. The fact is that the developer of the new product will have to undergo a difficult period of adaptation and search for consumers. However, at the moment, no one has had such circumstances anyway, so the matter has not reached the buyers!
For example, here are two companies, Leyden Energy and A123 Systems, that created new, quite promising technologies that never made it to the market. Why? The reason turned out to be banal: they didn’t meet the funds! You can also recall a couple more promising “battery startups” - Seeo and Sakti3. They were bought by third-party companies, and the transaction amounts were much lower than those that the first investors initially hoped for.
Electronics giants like Samsung, LG and Panasonic actually encourage upgrading the technology they already have rather than building a battery from scratch. So, at the moment, these manufacturers are optimizing lithium-ion electronic storage devices developed back in the 70s of the last century. We can only hope that the “miracle graphene” will still be able to make serious adjustments to these circumstances.
And much more
There are other applications of graphene, such as additives in concrete, antibacterial fabrics, filtering and adsorbing graphene materials, and more. The use of graphene in field-effect transistors and in lasers as saturable absorbers to implement passive self-mode locking during the generation of ultrashort pulses is being studied. Since all these applications have been developed in just a few years, it can be said that a more detailed study of the properties of graphene and the effects observed in it is necessary to fully unlock the potential of this carbon nanomaterial, which is predicted to be very large.
History of discovery
Graphene is a plate that is a crystal lattice of two-dimensional carbon crystals. The author of the new material, scientist Wallace, noticed the unusual properties of graphene in 1947. He argued that the substance had similar characteristics to metals.
The impossibility of obtaining carbon in its pure form in those days was due to the lack of proper equipment. With the advent of nanotechnology in 2004, scientists Novoselov and Geim obtained this material. Immigrants from Russia working at the University of Manchester were awarded the Nobel Prize for graphene.
Q-carbon
Among the recently discovered forms of carbon is the so-called Q-carbon. It was first synthesized by American materials scientists from the University of North Carolina in 2015. Scientists irradiated amorphous carbon using a powerful laser, locally heating the material to 4000 degrees Celsius. As a result, approximately a quarter of all carbon atoms in the substance adopted sp2 hybridization, that is, the same electronic state as in graphite. The remaining Q-carbon atoms retained the hybridization characteristic of diamond.
Q-carbon
ncsu.edu
Share
Unlike diamond, graphite and other forms of carbon, Q-carbon turns out to be ferromagnetic, such as magnetite or iron. At the same time, its Curie temperature was about 220 degrees Celsius - only with such heating did the material lose its magnetic properties. And by doping Q-carbon with boron, physicists obtained another carbon superconductor, with a transition temperature of about 58 kelvin.
2D world
Graphene-based materials will change the world, because they themselves are a different world, two-dimensional. Although this will not be a revolutionary innovation, but, as Andrey Geim emphasizes, a slow diffusion of the material into our everyday life. While painting these pictures of a bright future, we need to answer the inevitable question: where is Russia, the homeland of the Nobel laureates who received the prize for advanced experiments with graphene, in all this? If we have certain results in the field of fundamental research, then we cannot yet talk about leadership in the field of applied developments, although it is specific technological solutions that will form the main part of the graphene market in the near future. The next article in the series will tell you what will help our country not miss its place in the sun of graphene technologies.