Historical information
The chemical element has been known to people since ancient times. One of the first methods of metal extraction mastered by man was the smelting of lead. The first archaeological finds confirming this were lead beads found from the times of Çatalhöyük (modern Turkey). The items date back to 6400 BC.
The oldest lead figurine of a girl in long clothes was excavated in Egypt. It dates back to the time of the first dynasty of the pharaohs (3000 BC).
Lead pipes made up the ancient Roman water supply system. In the Ancient Roman Empire, up to 80 thousand tons of this metal were smelted annually. In Rus', since ancient times, lead has been used as roofing for cathedrals and churches.
Since time immemorial, the low melting point of lead has made it possible to obtain the metal and manufacture products of any shape from it.
Note! Over the course of 20 years, the Industrial Revolution since 1840 has raised the volume of annual lead smelting in the world from 100 to 250 thousand tons per year.
Being in nature
Soldering aluminum
Plumbum is usually not found in its pure form. It is found in more than 100 different minerals in the form of intermetallic agglomerates. Lead is present in uranium and thorium veins. Large accumulations of lead-zinc ores have been discovered and are being developed in Transbaikalia and the Primorsky region. Lead is mined in various deposits in the Urals and Norilsk.
The largest deposit with a high content of lead is located in the uranium ores of the Kohistan Ladakh arc (northern Pakistan).
Fossil lead
Receipt
How to solder a wire without a soldering iron
The raw materials for lead extraction are rocks that include helenite. The heavy metal smelting process consists of several phases. From the initial raw material, a concentrate containing from 40 to 70 percent plumbum is isolated by flotation. Next, manufacturers take different paths.
One of the ways to transform the product into werkbley (blank lead) is smelting using the regeneration method. Another method is that the metal is restored from the oxide by melting the raw material in a water jacket heater.
The resulting werkley containing 90% lead is purified from copper. Then arsenic and antimony are removed by alkaline refining. Then silver and zinc are isolated. The effects of magnesium and calcium exclude bismuth. As a result, lead with a purity of 99.8% is obtained.
Global lead production based on research by international organizations for 2005
| Manufacturer country | Volume, kilotons |
| Countries of Europe | 2220 |
| China | 1430 |
| Russian Federation | 1120 |
| South Korea | 650 |
| Kazakhstan | 570 |
| Ukraine | 410 |
Technological properties and characteristics
The characteristics of the metal can be represented by the list:
- Lead density and mass;
- Lead smelting temperature;
- Mechanical properties;
- Corrosion resistance.
Lead density and mass
The density of the metal is 11342 kg/m3. This means that a metric cube of lead weighs 11.342 tons. Its large specific gravity allows it to be used as payloads in various devices.
Lead smelting temperature
Molten metal in its pure form has a temperature of about 400 degrees. In this state, lead has fluid properties. The casting qualities make it possible to pour lead in a liquid state into molds of complex configurations.
Pouring a mold with lead
The metal boils when heated to 1750 degrees. During boiling, volatile fumes arise in the form of lead dust and oxide vapors, which can cause severe poisoning to the human body.
Mechanical properties
The chemical element has softness and ductility, which allows cold rolling to achieve the state of thin foil. Cold deformation does not affect the change in mechanical properties.
Corrosion resistance
The chemical inertness of the element is close to that of noble metals. In the air, plumbum practically does not corrode. The rapidly forming oxide film on the surface of lead puts an insurmountable barrier to corrosion processes.
Aggressive environments for lead are hydrogen sulfide, coal anhydrite and sulfuric acid. Under their influence, the metal is actively destroyed.
Security measures
When melting lead, one should take into account the increased fluidity of the liquid material, which causes burns if it comes into contact with the skin. Melt spilled on clothing impregnates the fabric, which increases thermal damage. If a heated substance gets on wooden or plastic surfaces, a fire may occur, so work should be performed at a distance from interior items and using protective clothing and gloves that are resistant to impregnation with liquid lead.
Please note that it is strictly forbidden to cool molten metal with water. When liquid gets in, there is an intense release of small hot particles, causing burns and leading to damage to interior items in the room or to a fire. When carrying out work, it is necessary to protect your face with a mask and goggles, and also use a personal breathing device or respirator.
If melting is carried out in an enclosed space (for example, at home or in a garage), then forced ventilation must be provided. Inhalation of metal fumes leads to a gradual accumulation of lead in the bones. The maximum permissible concentration of vapors in the atmosphere is 0.003 mg/m³, which once again proves the high toxicity of the metal and its compounds. Separately, it is necessary to mention alloys with cadmium, the vapors of which cause cancer and affect the human nervous and skeletal systems.
Application areas of lead alloys
Lead compounds are divided into high-alloy and low-alloy alloys. The former are formed by adding a large number of chemical elements that provide high strength, abrasion resistance and low shrinkage at a lower melting point.
Low alloy lead compounds are produced by small inclusions of substances such as tin, antimony, copper and cadmium. This achieves increased resistance of the alloy to corrosion processes in a polluted atmosphere, inorganic acidic environment.
The alloys are used in acid and alkaline batteries, as shells for both high-power and low-voltage cables. Compounds of antimony or copper with lead are used for the production of pipelines, sheet lining of various devices and protective mats against radiation damage.
Tin Characteristics
Melts at 232 °C, boils at 2600 °C, perfectly alloys with various metals, and, due to its high ductility, can be forged well. Soldering tin is used as solder because it wets metals well. Industrial production of tin is much more difficult than lead, so it is much more expensive.
Unlike lead, tin looks much more attractive. This silvery-white metal is safe for human health. Tin is often used to cover the surfaces of metal products in places where they come into contact with food: dishes, tin plates, food foil and others. However, tin dust and fumes can cause hazardous effects on the human body if inhaled. In addition to the production of food containers, tin is widely used in various solders and other alloys, for example, in antifriction and bearing alloys. This material is much lighter than lead, its density is 7.3 g/cc.
https://youtube.com/watch?v=I33GCWrUJFE
Tin is polymorphic, meaning it can exist in various modifications depending on temperature. At temperatures below 13 °C, white tin (β-modification) turns into gray tin (α-modification). As a result of this phase transition, the shiny pewter pieces crumble into a gray powder. Moreover, upon contact with the powder, white tin becomes infected with it and turns into gray. This phenomenon is called the “tin plague.”
According to some reports, it was the main reason for the death of Robert Scott's expedition to the South Pole. Kerosene, stored in intermediate warehouses, leaked from canisters sealed at the seams with tin, which crumbled into powder in the frosts of the Antarctic. Thus, the expedition members were left almost without fuel.
Home and industrial methods
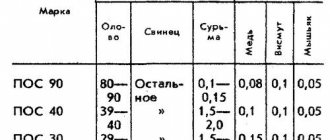
Without tin-lead solders (PLS), the existence of such an industry as radio engineering is impossible. Many industrial products contain POS coatings.
Tin-lead solders
The industry supplies the market with solder product:
- cast pigs;
- wire;
- foil tape;
- solder tubes with flux;
- powder or paste.
Alloys containing 90% tin and 10% lead are used for soldering products, which are then electroplated with gold or silver. The melting point of pure tin is 2310 C. Therefore, the solder will melt when heated to 2200 C.
Tubular solder with flux
Tin-lead POS with a predominance of tin in its composition (61%) has a lower melting point - 191%. POS 61 is used to cover contact groups in various devices; it is also used to process thin wire for windings of electric motor armatures and transformer coils.
Important! Taking into account the temperature at which tin melts, the % lead content in the alloy is regulated. This achieves a comfortable temperature regime, at which the tin-lead solder quickly turns into a liquid state.
POS 30 melts at 256 degrees. The compounds are less durable than products with a higher tin content.
10 percent solder is far from the temperature threshold at which tin melts. Therefore, POS 10 is used as a durable material for tinning large metal surfaces.
Melt preparation and pouring
In industrial conditions, the melt is prepared in special crucibles, which are placed in electric furnaces (equipment equipped with electronic measuring equipment that maintains the desired melting mode).
In radio engineering production, special heating baths are used to prepare solder for printed circuit boards of radio circuits.
In workshops and at home, solder is melted with a soldering iron tip. To prepare a large volume of molten metal, it is placed in a copper vessel on an electric stove. The alloy in the form of scrap is loaded into the melting bath gradually, as the next layer of metal melts.
Fishing varnishes
Avid fishermen at home cast fishing sinkers and spoons by pouring molten tin into clay molds. The spoons are then coated with waterproof varnishes.
Interesting. Fishing varnish is used to protect against the appearance of oxides on various figurines and other products.
Fishing varnish
Safety precautions
Molten lead can cause significant injuries and burns. The drop will instantly burn through the clothing and land on the exposed skin. The liquid form of lead can leak onto flammable objects and cause a fire in the room. If water penetrates into the liquid melt, a sharp flash occurs with the spread of small splashes of metal over the entire area. Such inclusions can get on the skin and eyes, which is painful and dangerous for human organs. Thus, while working, it is imperative to use clothing that covers the entire body, including sleeves, and wear a hat. The fabric must be highly fire-resistant and heat-resistant. You must wear a mask and safety glasses on your face.
The room in which smelting is carried out must be well ventilated, due to the toxicity of lead evaporation. If a mask is not available, use a cotton-gauze bandage. If lead enters the body, it can provoke and aggravate a number of painful processes, accumulating in organs, the element causes acute poisoning.
Methods for getting rid of oxide
When exposed to air, lead products become covered with an oxide film. This is the result of the ionic interaction of oxygen and lead atoms. The oxide becomes not only protection against an aggressive environment, but also a barrier to electric current.
Important! Mechanical cleaning will not bring the desired result. The film will recover quite quickly. Sunflower oil, graphite grease or varnish can help get rid of oxides.
At home, the product is placed in a vessel with sunflower oil for about five minutes. After which it is removed from the vessel and allowed to dry.
In industrial conditions, graphite lubricant is used. The lead surface treated with the product retains its shiny appearance for a long time.
Preparing to smelt lead
First you need to find a container. It would be great if the handle of the vessel was made of some heat-resistant material. For this purpose, you can use an old coffee pot or kettle.
The material can also be melted in an outdated container made of cast iron, using a deep and long spoon for pouring.
If there is no suitable container nearby, then you can use an ordinary tin can. However, here you should use pliers, which will be used to remove the hot pan from the flame and pour the material into the mold.
Do not forget that you need to be extremely careful while working. To simplify the procedure, you can make a small groove on one side of the jar. In this case, the red-hot metal will pour out in a thin stream clearly into the required place.
The material, cleared of impurities, can be crushed so that it melts as quickly as possible. The container must be securely placed over the burner and heated properly. This must be done in order to rid the surface of excess impurities and moisture.
Melting procedure
There is no need to try to melt all the prepared lead at once, because only the very bottom layer will interact with the hot surface of the container.
Melt two or three pieces first to form a puddle, then gradually add new material. This will give you the opportunity to make your work area more voluminous.
After melting, a layer of debris, impurities and slag must be removed from the surface of the metal. Pouring must be done into a heated mold. Lead is also characterized by rapid solidification. The material quickly loses its fluidity, becomes thicker, and therefore cannot completely fill the mold.