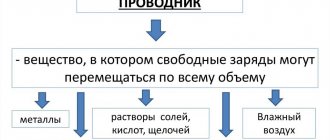
Those who remember well the school curriculum for the Chemistry course will immediately answer the question of what electroplating is. For those who have forgotten a little, let us remind you that this is a branch of electrochemistry, the so-called process when a metal coating is applied to almost any product. This process is also used on an industrial scale, for example, both in the galvanizing or chrome plating of metal products, and in the manufacture of decorative items.
The process of deposition of electrolytes onto the desired surface is quite complex and requires compliance with safety precautions and certain home processing skills. Electroplating at home will not allow you to increase the strength of a metal product (this requires industrial capacity), but it can be used to decorate individual items.
Galvanic laboratory at home
To organize the process you will need:
- Do-it-yourself galvanic bath - a jar (made of glass or durable plastic, large enough to fit the product being processed, heat-resistant) with an electrolyte solution.
- A wire divided into an anode (“plus”) and a cathode (“minus”). In this case, the anodes must be larger in area than the product being processed. They conduct current into the electrolyte and replace the loss of metal in it, which will be deposited on the galvanized product.
- Weighing equipment, such as precision electronic scales.
- A DC power source with voltage regulation, a household outlet will not work.
- Electric stove with mandatory temperature control.
The process of applying galvanic coating at home is quite simple: dilute the electrolyte in a container, heat it, immerse the anodes connected to the “plus” in it, secure the galvanized product at a distance (in our case, the cathode), which is connected to the “minus”. When connected to a current source, the metal from the electrolyte begins to settle on the “minus”, that is, on the product.
Process technology
Electrolytes are substances that can release ions – charged particles – under the influence of an electric current. This is what the principle of galvanization is based on. In our case, chromic anhydride will be used as an electrolyte. Released particles that will be deposited on the workpiece, forming a film - chromium molecules.
To chrome a part at home, it must be immersed in a bath of solution and connected to the negative wire. The positive anode is immersed in the electrolyte. Under the influence of current, the molecules in the electrolyte will begin to move. Positively charged to the minus (cathode), negative - to the plus. Moreover, some of the molecules form a film, and some will penetrate into the top layer, as a result of which chromium is firmly fixed on the surface. This makes galvanizing significantly different from conventional painting.
In a similar way, not only chrome plating is carried out, but also nickel plating, coating of products with copper and zinc. The processing principle will be the same in any case. The thickness of the spraying will depend on the current strength, heating temperature, processing time, and type of metal.
It is also possible to carry out chemical chrome plating at home. No special equipment is required here. The formation of a metal film on the surface in this case occurs due to chemical reactions in which sodium hypophosphite serves as a reagent. But such a coating is less durable - it is used only for decorative purposes.
This is interesting: How to cook stainless steel semi-automatically in a carbon dioxide environment: video, tips
What is needed to prepare electrolyte?
How to make electrolyte at home? First, let's choose the right container for storage: it should be a container made of an inactive substance (glass or plastic), durable, and tightly closed with a lid to prevent oxygen from entering the electrolyte.
Advice! A do-it-yourself electroplating rectifier can easily be made from a regular car battery.
Chemistry is an exact science. Each substance used will have to be measured to the nearest hundredth of a gram. You will need high-quality weighing equipment, preferably electronic. If there is no opportunity or desire to buy scales, take small change from the Soviet period; coins then had an accurate weight.
The most difficult thing for an ordinary citizen to obtain is the acquisition of reagents for the production of electrolyte. Many substances are prohibited for sale to individuals, only to industrial enterprises with special permission. Dangerous reagents will not be sold to ordinary people!
On video: Current 60A at home or homemade electroplating.
How to prepare the product
Having collected the weight of the necessary components, prepared the containers, heating system and power source, we proceed to preparing the product that we want to process.
In order for the metal from the electrolyte to settle in an even layer on the object, it must be very well cleaned, otherwise the galvanic coating at home will turn out uneven and fragile. Some items will simply need to be degreased, others will require cleaning with sandpaper and grinding to remove corrosion and “burrs” from the surface.
Important! High-quality degreasing is provided by acetone solution, alcohol and even gasoline.
Steel products are kept for several minutes in a solution of sodium phosphate heated to 90 degrees. Non-ferrous metals are also degreased in sodium solution, but without heating.
General idea of electroplating
Galvanic coating can be either technological or decorative-protective. It is a thin surface layer of metal that has a good aesthetic appearance (gold, silver) or anti-corrosion properties (zinc, copper) on metal or plastic products.
In general terms, metal electroplating at home looks easy.
This is interesting: Features of welding aluminum with an inverter at home
Safety precautions
Before you begin the galvanizing process, do not forget about safety precautions. Do-it-yourself electroplating does not involve manipulation, for example, in the kitchen. We are talking more about a garage or barn, a non-residential place with good ventilation, where grounding can be organized.
Important! Don't get poisoned by toxic fumes ! Galvanization can cause real harm to health. Organize an exhaust hood and cover your face with a respirator mask.
Thick rubber gloves are required. Protect your eyes with glasses. Before starting manipulations, read special literature. If you experience any symptoms of discomfort, consult a doctor immediately.
Processing options
Nickel plating
Applying a nickel coating to metal objects is a simple process, as a result of which your products will receive a luxurious, shiny appearance and will become more resistant to rain and other phenomena.
You will need:
- Prepare an electrolyte for electroplating by mixing nickel sulfate, sodium, magnesium, sodium chloride (table salt) and boric acid. Check the pH, it should be in the range of 4-5.
- Warm up the electrolyte to 25 degrees.
- Place the product in the container and connect a current of 1.2 A/sq. dm.
- Approximate time is about half an hour.
The specified time depends on factors such as product size, current density and electrolyte temperature. The longer the time, the thicker the layer of applied nickel will be. When finished, rinse the item and polish with any polishing ointment.
On video: chemical nickel plating.
Chrome plating
One of the most popular ways to add strength and appearance to metal products is chrome plating. Although it will not be possible to achieve high strength at home, this requires a current density of 100 A/sq. dm., you can still apply a decorative coating.
The chrome coating is porous. Before using it, the item is coated with copper or nickel. But home chrome plating allows you to achieve a greater variety of shades, which is achieved by different electrolyte temperatures: the higher it is, the more shiny the coating will be.
The process of chrome plating at home is as follows:
- Anodes made of lead, tin and antimony (85%/11%/4%).
- Immerse the product in the electrolyte at the desired temperature and wait about half an hour.
- Rinse in a weak solution of baking soda, dry, polish.
On video: decorative chrome plating at home.
Copper plating
Coating metal surfaces with copper at home is used to create a layer that will subsequently conduct current, or to protect against corrosion.
It is impossible to do copper electroplating on ferrous metals at home, since it uses deadly cyanides. Initially, steel and cast iron items must be nickel-plated, and then galvanized by copper plating using copper sulfate salts diluted in sulfuric acid. Coating aluminum products with copper will require initial cleaning of the latter from oxide in an electrolyte containing sulfuric acid, and then galvanize it in the same way as steel.
On video: galvanic copper plating.
Galvanizing
The easiest home-made galvanization method is zinc treatment. It is used to protect metal objects (electrically conductive and non-electrically conductive) from corrosion. When galvanizing, a zinc plate corresponding in area to the object being galvanized is immersed in an electrolyte as an anode and connected to a current source.
The electrolyte contains: zinc sulfate (200 g), ammonium sulfate (50 g), sodium acetate (15 g) per 1 liter of water. In about half an hour, the anode will dissolve and its molecules will cover the object being treated with a dense layer.
On video: galvanizing metal at home.
Brass plating
The most decorative method of electroplating is brass plating (applying a film of an alloy of copper and zinc). Brass-coated products are used for furniture fittings, as door handles, etc. Brass gives objects a noble golden color and rich shine.
The electrolyte for brass plating must contain copper and zinc salts dissolved in a cyanide solution. This type of galvanization is also not recommended for use at home due to the possibility of cyanide poisoning.
No matter how exciting the galvanization process is, repeating it at home without prior preparation is not recommended - it can be life-threatening. The equipment costs money, and you simply cannot purchase some of the reagents necessary for the production of electrolytes. Starting a process, for example, for chrome plating one part is not worth it - it will be cheaper to contact specialized enterprises.
Silvering and gilding
The electroplating of silver on products has not only a decorative purpose, it also protects against corrosion and forms an electrically conductive coating. As with copper, cast iron and steel are pre-plated with nickel and then silver-plated.
Electrolyte for silvering contains:
- silver chloride;
- potassium iron cyanide;
- soda ash;
- distilled water.
The electrolyte must be heated to a temperature of up to 20 degrees. High power is not required - 0.1 A/sq. is enough. dm. The anode will be a graphite plate, the size corresponding to the size of the galvanized product.
Gold electroplating is the most decorative method.
To do this, you will need a heated solution of gold in proportions of 5 g per 1 liter of water, mixed with potassium synoxide. You can also use a cold electrolyte, but then you will need 3 times more gold.
Be extremely careful - the fumes of hydrocyanic acid are extremely dangerous, both hot and cold. Do not neglect ventilation, do not allow it to come into contact with exposed skin. If possible, replace it with potassium iron sulfide.
Clean the product thoroughly beforehand. If it is made of ferrous metal, cover it with copper first, then gild it. To make gold “stick” better, dip the product in mercury nitrate.
On video: galvanic gilding of a silver spoon.
The main rule: be careful when using current - it should not be more powerful than 1 A/sq. dm. A stronger current will cause the gold to fall in black flakes to the bottom of the container, and the galvanized object will turn brown instead of gold. After the process is completed, the product is dried and polished using polishing ointment.
Galvanoplasty and galvanostegy
What is galvanoplasty? This is a method that is used to make exact copies of products, the copying method. It is used when it is necessary to make a copy of objects of the finest configuration - records, chips and circuits. Electroplating allows you to enhance the mechanical properties of one metal by applying a layer of another metal to it, for example, chrome and nickel plating of steel, nickel plating of copper, etc.
Galvanoplasty and galvanostegy are of a similar nature, differing only in the method of preparing the metal before processing. When performing electroplating, the metal surface must be as prepared as possible for adhesion to the applied metal. The electroplating method, on the contrary, involves the free separation of the applied metal.
Copper, nickel and silver are most often used for electroplating processes, and almost all types of metals are used in electroplating processes. Home electroplating is performed using the same equipment as other electroplating processes.
A large glass container is perfect for a galvanoplastic bath. Its dimensions depend on the size of the item being galvanized, since it should not be located too close to the anode plate.
Electroforming at home can be used to make copies of small-sized objects using molds pre-cast from low-melting metals.
Master class on galvanizing (1 video)
Items with galvanic coating (17 photos)
Protection of electroplating installations at home
As previously noted, providing reliable protection is the most important condition that will avoid many problems when carrying out galvanic procedures. If the galvanic bath was made of plastic, then practically no additional protection will be required. But if larger scale volumes of work are planned and the bath for the electrolyte is made of metal, it will be necessary to protect it from corrosion, destructive processes upon contact with solutions, as well as distortion of the electric field. This is not difficult to do - you will need to line the installation using sheet polymers by hot welding.
Author of the materials: Gordienko Anastasia Vadimovna Position: chief technologist of 6 Micron LLC Education: higher Experience in galvanizing: 11 years