Chemical current sources are devices and instruments that produce voltage during a chemical redox reaction. They are also called electrochemical, galvanic cells. Their basic principle of operation is based on the interaction of chemical reagents which, when reacting with each other, generate electricity in the form of direct current. This process occurs without mechanical or thermal influence, which are the main factors that play a superior role among other constant voltage generators. Chemical current sources, abbreviated as HIT, have long been used not only in everyday life, but also in production.
A little history of the creation of HIT
Back in the eighteenth century, the Italian scientist Luigi Galvani came up with the simplest element that chemically generated electric current. However, he was not only a scientist, but also a physicist, doctor, and physiologist. He was interested in and conducted experiments that were aimed at studying the reactions of animals to external stimuli. Like everything ingenious, the first chemical source of energy was obtained by Luigi absolutely by accident, during numerous experiments on frogs. After attaching two metal plates to the frog's leg muscle, muscle contraction was observed. Galvani considered this a nervous reaction to an external stimulus and outlined this in the results of his research, which fell into the hands of another great scientist, Alessandro Volta. He laid out his theory about the occurrence of tension as a result of a chemical reaction that arose between two metal plates in the environment of the muscle tissue of a frog.
The first chemical source of electric current was a container with a salt composition, into which two plates of different materials were immersed. One is copper, the other is zinc. It was this device that in the future, and more specifically in the second half of the nineteenth century, was used in the invention and creation of a manganese-zinc cell within which was the same salt electrolyte.
Research work on the topic: “Chemical source of electric current” (grades 7-9)
Municipal budgetary institution of additional education of the Pervomaisky district of the city of Rostov-on-Don “House of Children's Creativity”
Topic: “Chemical source of electric current”
Bobylchenko Valery Yurievich
Additional education teacher
MBU DO DDT Pervomaisky district
2019
Introduction
Chemical power sources have become a part of our lives for many years. In everyday life, consumers rarely pay attention to the differences between the chemical power sources used. For him, these are batteries and accumulators. They are typically used in devices such as flashlights, toys, radios or cars. In the case when the power consumption is relatively high (10Ah), batteries are used, mainly acid batteries, as well as nickel-iron and nickel-cadmium. They are used in portable electronic computing machines, wearable communications equipment, emergency lighting, etc.
Due to a number of circumstances, chemical generators of electrical energy are the most promising. Their advantages are manifested through parameters such as high energy yield; noiselessness and harmlessness; possibility of use in any conditions, including in space and under water, in stationary and portable devices, in transport, etc.
In recent years, such batteries have been widely used in backup power supplies for computers and electromechanical systems that accumulate energy for possible peak loads and emergency power supply of vital systems.
Relevance of the topic
In technology and everyday life, the number of devices and devices that require autonomous, small-sized, lightweight and reliable current sources is constantly growing. A galvanic cell, or, more simply put, a battery, is not capable of providing enormous power, but it is impossible to do without it in cases where a regular network is not available or is not advisable.
Target
Make a simple galvanic cell
Tasks
— Familiarization with the history of the creation of the first galvanic cells.
— Study the structure and principle of operation of galvanic cells.
— Galvanic cells today.
Chemical current sources: main characteristics
For more than two centuries, humanity has been using the energy of chemical reactions between various substances to produce direct current.
Principle of operation
The redox reaction that occurs between substances that have the properties of an oxidizing agent and a reducing agent is accompanied by the release of electrons, the movement of which forms an electric current. However, in order to use its energy, it is necessary to create conditions for the passage of electrons through the external circuit, otherwise it is released into the external environment by heat when simply mixing an oxidizing agent and a reducing agent.
Therefore, all chemical current sources have two electrodes:
- anode where oxidation occurs;
- a cathode that performs the reduction of a substance.
The electrodes are placed at a distance in a vessel with an electrolyte - a substance that conducts electric current due to the processes of dissociation of the medium into ions.
The principle of converting chemical energy into electrical energy
A galvanic cell is a chemical source of electric current based on the interaction of two metals and/or their oxides in an electrolyte, resulting in the generation of electric current in a closed circuit. Thus, in galvanic cells, chemical energy is converted into electrical energy.
Prerequisites for the emergence of galvanic cells. In 1786, the Italian professor of medicine, physiologist Luigi Aloisio Galvani discovered an interesting phenomenon: the muscles of the hind legs of a freshly opened frog corpse, suspended on copper hooks, contracted when the scientist touched them with a steel scalpel. Galvani immediately concluded that this was a manifestation of “animal electricity.”
After Galvani's death, his contemporary Alessandro Volta, being a chemist and physicist, would come to the unequivocal conclusion that current appears in the circuit due to the presence in it of two conductors of different metals placed in a liquid, and this is not “animal electricity” at all. , as Galvani thought. The twitching of the frog's legs was a consequence of the action of current generated by the contact of different metals (copper hooks and a steel scalpel).
Volta will show the same phenomena that Galvani demonstrated on a dead frog, but on a completely inanimate homemade electrometer, and will give in 1800 a precise explanation for the occurrence of current: “a conductor of the second class (liquid) is in the middle and is in contact with two conductors of the first class from two different metals... As a result, an electric current arises in one direction or another.”
In one of his first experiments, Volta dipped two plates - zinc and copper - into a jar of acid and connected them with wire. After this, the zinc plate began to dissolve, and gas bubbles appeared on the copper steel. Volta suggested and proved that an electric current flows through a wire.
This is how the “Volta element” was invented - the first galvanic cell. For convenience, Volta gave it the shape of a vertical cylinder (column), consisting of interconnected rings of zinc, copper and cloth, soaked in acid. A voltaic column half a meter high created a voltage that was sensitive to humans.
This invention was subsequently used by other scientists in their research. For example, in 1802, Russian academician V.V. Petrov constructed a Voltaic column of 2100 elements to produce an electric arc. In 1836, English chemist John Daniel improved the Voltaic element by placing zinc and copper electrodes in a solution of sulfuric acid. This design became known as the "Daniel element". In 1859, French physicist Gaston Plante invented the lead-acid battery. This type of cell is still used in car batteries today. In 1865, the French chemist J. Leclanchet proposed his galvanic cell (Leclanchet element), which consisted of a zinc cup filled with an aqueous solution of ammonium chloride or other chloride salt, into which was placed an agglomerate of manganese(IV) oxide MnO2 with a carbon conductor. A modification of this design is still used in salt batteries for various household devices. In 1890, in New York, Conrad Hubert, an immigrant from Russia, creates the first pocket electric flashlight. And already in 1896, the National Carbon company began mass production of the world's first dry cells, Leclanche "Columbia".
Experiments by V.V. Petrova
Vasily Vladimirovich Petrov (1761 - 1834) - Russian experimental physicist, self-taught electrical engineer, academician of the St. Petersburg Academy of Sciences. Founder of Russian electrical engineering [1].
After Alessandro Volta's discovery of a device capable of creating a continuous flow of electrical charges, scientists were able to conduct new experiments with electricity. In St. Petersburg, experiments with the Voltaic Column were carried out by Vasily Vladimirovich Petrov, professor of physics at the Medical-Surgical Academy. He ordered 100 zinc and 100 copper mugs, each 10 inches in diameter. Each circle weighed over a pound. From these, Petrov composed a Voltaic pillar, using paper mugs soaked in an aqueous solution of ammonia instead of cloth pads. However, the power of the device did not satisfy Petrov. For the experiments he planned, this battery was rather weak, and the scientist ordered another - “especially a huge battery, sometimes consisting of 4200 copper and zinc circles.”
Battery V.V. Petrova
In this battery, Petrov did not arrange the mugs in a column. A column of 4200 circles was, according to Petrov’s calculations, 40 feet high, that is, more than 12 meters. Handling such a pillar would be difficult; the ceilings in the laboratory would have to be broken, and the battery would rise above the roof of the building, like a factory chimney. And most importantly, the scientist was afraid that under the weight of the column, moisture would be squeezed out of the gaskets at the bottom of the battery, and the expected result would not be achieved.
Petrov ordered mahogany boxes divided into eight compartments. He doused the inner walls of the box and all the partitions with molten sealing wax. When the sealing wax hardened, it formed a hard, completely waterproof crust that served as excellent insulation.
Petrov placed 525 copper and zinc mugs in each compartment. Petrov connected all sections of his battery with insulated wires, using silk, sealing wax, wax, and varnishes for insulation. This was a major technical innovation. But none of the scientists understood then how important it is to carefully insulate the conductors. Petrov proved that only a reliably insulated battery is capable of delivering the strongest current.
With the help of his voltaic column, Petrov created an electric arc - he discovered one of the types of electric discharge - an arc discharge.
Galvanic cells today
Galvanic cells today are called batteries. Three types of batteries are widely used: salt (dry), alkaline (they are also called alkaline, “alkaline” translated from English as “alkaline”) and lithium. The principle of their operation is the same as described by Volta in 1800: two metals interact through an electrolyte, and an electric current arises in an external closed circuit.
The voltage of the battery depends both on the metals used and on the number of elements in the “battery”. Batteries, unlike accumulators, are not capable of restoring their properties, since they directly convert chemical energy, that is, the energy of the reagents that make up the battery (reducing agent and oxidizing agent), into electrical energy.
The reagents included in the battery are consumed during its operation, and the current gradually decreases, so the effect of the source ends after the reagents have reacted completely.
Salt batteries
Manganese-zinc cells, which are called salt batteries, are “dry” galvanic cells that do not contain a liquid electrolyte solution.
The zinc electrode (+) is a glass-shaped cathode, and the anode is a powdered mixture of manganese dioxide and graphite. Current flows through the graphite rod. The electrolyte is a paste of ammonium chloride solution with the addition of starch or flour to thicken it so that nothing flows.
Typically, battery manufacturers do not indicate the exact composition of the salt cells, however, salt batteries are the cheapest, they are usually used in those devices where power consumption is extremely low: watches, remote controls, electronic thermometers, etc.
Alkaline (alkaline) batteries
An alkaline battery is a manganese-zinc voltaic battery that uses manganese dioxide as the cathode, powdered zinc as the anode, and an alkali solution, usually in the form of potassium hydroxide paste, as the electrolyte.
These batteries have a number of advantages (in particular, significantly higher capacity, better performance at low temperatures and at high load currents).
Alkaline batteries, compared to salt batteries, can provide more current for a longer period of time. A higher current becomes possible because zinc is used here not in the form of a glass, but in the form of a powder that has a larger area of contact with the electrolyte. Potassium hydroxide in the form of a paste is used as an electrolyte.
It is thanks to the ability of this type of galvanic cells to deliver significant current (up to 1 A) for a long time that alkaline batteries are most common today. They last 1.5 times longer than salt ones if the discharge is low current.
Lithium batteries
Another fairly common type of voltaic cell is lithium batteries - single, non-rechargeable voltaic cells that use lithium or its compounds as the anode. Thanks to the use of alkali metal, they have a high potential difference.
The cathode and electrolyte of a lithium cell can be very different, so the term "lithium cell" combines a group of cells with the same anode material. For example, manganese dioxide, carbon monofluoride, pyrite, thionyl chloride, etc. can be used as a cathode.
Lithium batteries differ from other batteries in their long service life and high cost. Lithium cells are widely used in modern portable electronic equipment: to power clocks on computer motherboards, to power portable medical devices, wristwatches, calculators, in photographic equipment, etc.
Practical part
A number of metal stresses are necessary in our case. We, like Alessandro Volta, will know that the further the metals are separated from each other, the greater the voltage can be obtained.
In our experiments, like the classics, we used copper and zinc. When the plates are immersed in the electrolyte, between it and the zinc plate, a chemical reaction occurs, as a result of which negative charges accumulate on the plate and it becomes negatively charged. As a result of the reaction occurring in the galvanic cell, the zinc electrode gradually dissolves.
On the copper electrode, during the operation of the galvanic cell, tiny hydrogen bubbles are formed, insulating the surface of the copper from the electrolyte. The phenomenon is called gas polarization; in a galvanic cell it is harmful and is being combated. To remove the evolved hydrogen, hydrogen-binding substances called depolarizers are introduced into the electrolyte. Their role is often played by manganese compounds and copper sulfate. In simple experiments, you can use pharmaceutical potassium permanganate.
Devices and materials.
To assemble galvanic cells, wire, wire, or foil can be used as copper electrodes. Zinc can be extracted from dry elements and galvanized products can be used. Instead of zinc, you can try using an aluminum or iron electrode. Table salt for the electrolyte, some soft wire. You definitely need a voltmeter or multimeter, wire cutters, and scissors. Non-metallic containers of suitable size can be used as vessels. Glass cups are more convenient than light plastic cups - they are heavier, more stable, and more difficult to knock over. It is very good if you can find a low-current, low-voltage load - a quartz watch. “High-voltage” battery made of wire and self-tapping screws.
Initially, we assembled such a battery (see figure). The “classical” pair of metals – copper-zinc – is used here. The idea is to use a galvanized bolt as a zinc electrode. It is clear that such an element is not designed for long-term operation - a thin layer of zinc will quickly dissolve, however, for a short-term experiment this is not important. Wire is used as a copper electrode - also a widely available material; in addition, it is very convenient to install elements in a battery - all elements are connected in series - plus one to the minus of the next. In this case, the voltage is summed, the current remains the same.
Experience 1.
Battery assembly - cells from boxed chocolates were used as a container. After installing the electrodes on the walls between the cells, fill the containers with electrolyte. We used a solution of table salt - a heaping tablespoon per 0.5 liter of warm water.
Experience 2.
Instead of zinc, lead was used as the electrode. The electrolyte was placed in a glass beaker. The voltage was low - 0.02 V.
Experience 3.
Graphite was used instead of lead. Voltage is more than 1V. Hydrogen was released on the graphite in the form of bubbles.
Experience 4.
Galvanized bolt and copper. Voltage is less than 1 V.
Experience 5.
Consistently increase the number of glasses. As a load, we alternately connect a quartz clock and an LED, observing the polarity.
Conclusion
Practice places very diverse demands on modern galvanic cells. As a result of the ever-increasing and highly diverse demand for galvanic cells, scientific research aimed at developing new and improving old types of cells has recently expanded again.
Galvanic cells as sources of electrical energy have significant advantages: they can be of various sizes and shapes, do not have macroscopically moving parts subject to wear, are relatively light and autonomous, are little sensitive to vibration and temperature fluctuations, operate silently, and are well regulated. Their efficiency is quite high (up to 90%), since the conversion of chemical energy into electrical energy occurs in them without an intermediate thermal stage, and electrode processes in some cases are close to reversible.
Bibliography
1. Electrical reference book. In 3 volumes. T.2. Electrical products and devices/under general. ed. professors of Moscow Power Engineering Institute (editor-in-chief I.N. Orlov) and others. 7th ed. 6 rev. and additional M.: Energoatomizdat, 1986. 712 p.
2. Bagotsky V.S., Skundin A.M. Chemical current sources. M.: Energoizdat, 1981. 360 p.
3. Crompton. T. Primary current sources. Moscow. "World". 1986
Electronic resources:
1. Andrey Povny https://electrik.info/main/school/1267-galvanicheskie-elementy-ustroystvo.html
2. https://vashtehnik.ru/enciklopediya/galvanicheskij-element.html
3. https://school-science.ru/5/11/34665
4. https://mirznanii.com/a/321369/galvanicheskie-elementy
Operating principle
Power supplies 24 and 12 Volts
The device that produces electric current contains two electrodes that are placed between the electrolyte. It is at their border of contact that a small potential appears. One of them is called the cathode, and the other the anode. All these elements together form an electrochemical system. During a redox reaction between the electrodes, one element donates tiny particles of electrons to the other. Therefore, it cannot happen forever, and over time, the properties of each element of this chain are simply lost. Electrodes can be presented in the form of metal plates or grids. After immersing them in a medium with an electrolyte, a potential difference arises between their terminals, which is called open circuit voltage. Even if at least one of the electrodes is removed from the electrolyte, the voltage generation process stops.
Composition of electrochemical systems
The following chemicals are used as electrolytes:
- Aqueous solutions based on alkalis, acids, salts, etc.;
- Non-aqueous ionic conducting solutions that are obtained by dissolving salts in inorganic or organic solvents;
- Solid compounds containing an ionic lattice, where one of the ions is mobile;
- Matrix electrolytes. This is a special type of liquid solutions and melts that are located in the pores of a solid non-conducting element - an electron carrier;
- Molten salts;
- Ion exchange electrolytes with unipolar conductivity system. Solids with a fixed ionogenic group of the same sign.
Principle of operation
A chemical source that produces direct current has a certain operating principle. The algorithm for generating electricity through chemical reactions between certain substances is quite simple to understand; a person far from chemistry or physics can figure it out.
Important! Due to the use of fairly aggressive types of substances that are part of such sources, it is prohibited to open the structures yourself. This can be harmful to health and life. Additionally, certain disposal is required.
A redox reaction occurs between the oxidizing agent and the reducing agent (electrolyte). As a result, electrons are released, which begin to move sequentially in a certain direction. It is thanks to the energy released as a result of the chemical reaction that the movement of elementary charged particles occurs.
This is how you get electric current, which you also need to be able to produce. If you do not create the necessary conditions for electrons to escape to the external circuit, then the substance will only release heat. To do this, you need to prepare two electrodes: the anode (where oxidation occurs) and the cathode (reduces the substance).
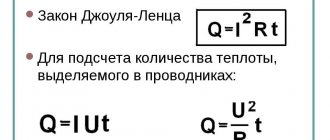
You might be interested in Electric current formulas
The principle of a chemical current source
The amount of electricity that is obtained as a result of the redox reaction depends on the following factors:
- volume and concentration of electrolyte;
- the material from which the electrodes are made;
- design of external electrical circuit.
There are several options for the most effective and used electrolytes with a certain concentration and mass.
Classification of galvanic cells and their selection
Basic concepts about relay protection
Generators of electric current resulting from a chemical reaction are divided into:
- Sizes;
- Design features;
- The method and reagent through which electricity is generated.
All elements that produce current during a chemical reaction are divided into:
- Chargers that can be repeatedly charged from a direct current source during operation are called batteries;
- Non-rechargeable, that is, disposable sources that, after the completion of a chemical reaction, simply become unusable and must be disposed of. Simply it is a galvanic cell or battery.
In order to select a source of electricity based on a chemical reaction, you need to understand its characteristics, which include:
- Voltage between anode and cathode when the circuit is open. This indicator most often depends on the selected electrochemical system, as well as the concentration and treated of all components;
- Source power;
- Current indicator;
- Capacity;
- Electrical indicators, that is, the number of charge and discharge cycles;
- Operating temperature range;
- The shelf life between the time an item is created and before it is put into use;
- Full service life;
- Strength, that is, protection of the housing from various mechanical damage and influences, as well as vibrations;
- Working position, some of them only work in horizontal positions;
- Reliability;
- Easy to operate and maintain. Ideally, there is no need for the slightest intervention in the work during the entire service life.
When choosing the right battery or accumulator, you must take into account its electrical ratings such as voltage and current, as well as capacity. It is this that is key to maintaining the functionality of the device connected to the source.
Modern chemical current sources and their applications
Introduction to peak and other electricity tariff zones
It is difficult for a person to live a modern life without these mobile energy generators, which he encounters throughout his life, starting with children's toys and ending with, say, a car.
The areas of application of various batteries and accumulators are so diverse that it is very difficult to list them. The operation of any mobile phone, computer, laptop, watch, remote control would be impossible without this portable and very compact device for creating a stable electrical charge. In medicine, sources of chemical energy are widely used to create any device that helps a person live a full life. For example, for hearing aids and pacemakers that can only operate from portable voltage sources, so as not to confine a person to wires. In production, entire battery systems are used to provide voltage for shutdown and protection circuits in the event of a loss of incoming high voltage at substations. And this food is also widely used in all vehicles, military and space technology. One of the types of common batteries is lithium electric current sources, since this particular element has a high specific energy. The fact is that only this chemical element turns out to have a strong negative potential among all substances known and studied by man. Lithium-ion batteries stand out among all other batteries in terms of the amount of energy generated and their small dimensions, which allows them to be used in the most compact and small electronic devices.
Classification
The most common types are galvanic cells and batteries. Almost everyone is familiar with them. But the classification of such devices is broader and also assumes the existence of fuel cells.
Current source classification scheme
Galvanic cells
The galvanic cell got its name in honor of the scientist Galvano, who discovered the wonderful possibility of producing electric current by creating a simple structure of electrolyte and electrodes. They are considered the first prototypes of modern devices for generating electricity through chemical reactions.
You may be interested in this Electric circuit designation
Chemical current sources - galvanic cells and batteries
Note! Currently, this device is more compact and safer to use; it is a regular battery. The peculiarity of the operation of such a device is that it is one-time use. After the final decomposition of the electrolyte into substances, it is impossible to recharge them for the following reactions.
Electric batteries
An electric battery is a more versatile version of the device that can be charged several times after losing its electrolyte charge. This feature is explained by the regeneration of substances that form the electrolyte.
Battery device
In this case, charging is carried out from an external (external) current source. Motorists often encounter this need to restore the reagent in batteries when charging the battery.
Fuel cells
An electrochemical fuel cell is a promising source that is quite important for creating comfortable and, in some situations, vital living conditions.
Thermal chemical source
The peculiarity of the operation of such an element is as follows. Each time, a certain portion of electrolyte is supplied to the electrodes, which, after discharge, is removed from the structure. For example, thanks to this operating principle, a backup current generator can produce electricity for 10-15 years.
Note! After the expiration date, operation can be extended if power is restored.
Methods for recycling chemical energy sources
The problem of recycling chemical voltage sources of different sizes is an environmental problem for the entire planet. Modern sources contain up to thirty chemical elements that can cause significant harm to natural resources, therefore entire programs have been developed for their disposal and specialized processing workshops have been built. Some methods make it possible not only to efficiently recycle these harmful substances, but also to return them to production, thereby protecting the environment. In order to extract non-ferrous metals from batteries and accumulators, entire pyrometallurgical and hydrometallurgical complexes have now been developed and used in civilized countries that monitor and care about the environment. The most common method for recycling waste chemical power sources is a method that works by combining these processes. Its main advantage is considered to be a high degree of extraction with a minimum amount of waste. This method of pyrometallurgical, hydrometallurgical and mechanical processing includes eight main stages:
- Grinding;
- Magnetic separation;
- Burning;
- Additional grinding;
- Isolation of large and small elements using screening;
- Water purification and leaching;
- Sulfuric acid leaching;
- Electrolysis.
Organizing the correct collection and disposal of chemical waste makes it possible to minimize the negative impact on both the environment and human health.