What is battery memory effect
The battery memory effect is a significant loss of battery capacity as a result of recharging a battery that has not fully discharged. The product seems to remember the previous capacity value at which it was set for recharging and during subsequent operation it delivers electric current only up to this level.
This process can be explained not by the appearance of cognitive abilities in an inanimate object, but as a result of an increase in the crystals of the active substance.
This “pathology” is most pronounced if some types of rechargeable batteries are installed for recharging until the available supply of electric current is fully discharged. If the battery is constantly used in this mode, the product will not only lose a significant amount of capacity, but may also completely fail.
To protect yourself from the need to replace batteries, you need to notice changes in the operation of such products in a timely manner. In general, the “symptoms” of different models manifest themselves almost identically, so it will not be difficult to timely determine the operation of the power source in a non-standard mode.
Despite the limited number of operating cycles of such devices, even severely damaged batteries, in many cases, can be restored with the help of special battery training.
Self-discharge
Standard Ni-MH batteries, like all other batteries, are subject to self-discharge. This means that their stored energy decreases over time.
The self-discharge rate of standard Ni-MH batteries is up to 40% within a month. At the same time, the battery loses 15...20% of its stored energy in the first day after charging and 10...15% of the remaining stored energy is lost during each subsequent month.
This means that standard Ni-MH batteries must be recharged immediately before use.
There are Ni-MH batteries with low self-discharge, usually with o or “LOW SelfDischarge”. Over the course of a year, their stored energy decreases by only 15%. These batteries come out of production fully charged and are ready for use immediately after purchase.
How does the memory effect manifest in batteries?
In order to eliminate the possibility of battery memory formation, it is recommended to first correctly determine the type of battery. Knowing the chemical composition of the electrolyte and electrodes, it is easy to determine the susceptibility of such elements to the effect of crystal growth.
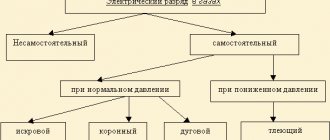
Ni-Mh. Almost all batteries that contain nickel are subject to the memory effect. Ni-Mh batteries are no exception to this rule.
It is enough not to completely discharge the battery once, so that the next time you use it, the capacity of the element will be significantly reduced. If a gadget or tool is used frequently, this effect may manifest itself in a noticeable decrease in the operating time of the device.
Ni-Cd. Nickel-cadmium products are the most susceptible batteries to memory effect. A decrease in capacity also manifests itself in the form of a decrease in operating time. This feature can manifest itself even with a short period of operation of the elements, especially in cheap models.
Li-Ion. Lithium-ion batteries are modern chemical sources of electricity and therefore have virtually no memory effect. Minor deviations in capacity, as a rule, are associated only with long-term operation of such products or with very intensive use
Li-Pol. Lithium polymer products also lack memory effect. Such products are ideal for devices that are used occasionally and are recharged long before the power is completely used up.
LiFePO4. Lithium iron phosphate cells are subject to a memory effect. Despite the fact that the decrease in capacity as a result of setting the product to recharge until completely discharged is not as significant as in Ni-Mh and Ni-Cd batteries, it is enough to violate the principle of complete energy consumption once to start a pathological process on the cathode of batteries of this type.
When it meets
Let's pay attention! Modern batteries do not have a memory effect. Accordingly, there is no point in constantly charging or discharging them. They work at full capacity and show stable operation, which is why such batteries for phones began to be used.
The following batteries are currently susceptible to memory effect:
- Metal-Hydride NI-MH.
- Nickel Kadim NI-Cd.
Such batteries are not so easy to find now, but they are still found on our territory.
How to prevent the memory effect
It is very easy to prevent the memory effect in batteries most susceptible to such pathologies. To do this, it is enough to always discharge the battery to 100% before installing an electrical source for recharging.
If for one reason or another it is not possible to fully consume electricity every time, then for prevention it is recommended to completely use up the reserve from time to time, and then fully charge the product with the current recommended by the battery manufacturer.
To reduce the likelihood of memory effect formation in nickel-cadmium and nickel-metal hydride batteries, it is recommended to “boost” them to the required capacity before using new products. For this purpose, it is enough to fully charge the product with a current that does not exceed the values set by the manufacturer.
Then discharge the device through a not very powerful consumer of electricity. Such training will allow you to fully unlock the potential of the device from the very beginning of operation and remove the initial formation of crystals on the internal contacts of the battery.
IMAX B6 Smart Charger
What should you remember when using Ni─MH batteries?
Despite the advantages of nickel-metal hydride batteries over nickel-cadmium batteries, they have a number of disadvantages. And they must be taken into account during operation.
To begin with, it should be noted that Ni-MH batteries are more expensive than Ni─Cd. True, technology does not stand still and the price of these types of batteries is gradually becoming comparable. In this case, we are talking about batteries of the common form factor AA (“finger”) and AAA (“little finger”). Nickel-cadmium batteries have a more pronounced “memory effect”, but, nevertheless, nickel-metal hydride batteries also face this problem. Nickel metal hydride batteries have fewer charge-discharge cycles. The first deterioration in their performance characteristics is observed after 200-300 charge-discharge cycles. This type of battery has a higher self-discharge compared to Ni─Cd batteries (about 1.5 times).
One more point is worth noting. Nickel-metal hydride batteries can deliver high current, but it is not recommended to set values greater than 0.5*C when discharging. This leads to a significant reduction in the number of charge-discharge cycles and a decrease in service life. For now, where high discharge currents are required, Ni─Cd batteries are still used.
Do not forget that a charger for Ni-MH batteries will work without problems with nickel-cadmium batteries, but not vice versa.
Which devices are most susceptible to the problem?
The memory effect is especially strong in portable devices that can be used for long periods of time. For example, screwdrivers used in non-electrified objects are charged to full capacity, even if the electricity supply is not used up.
This is due, first of all, to the fact that during the work process it will not be possible to install the device for recharging. A similar problem occurs if the wireless device is used periodically.
Workers, when there are breaks in using the device, connect it to the network via an adapter, which leads to a very rapid decrease in the efficiency of the power supply.
Are there special requirements for storing Ni-Cd and Ni-Mh?
When you need to preserve the standard characteristics of both types of batteries as much as possible, you should follow some recommendations from the manufacturers. We have included them in this list.
- • In a cool, dry place (self-discharge increases at high temperatures).
- • With any intermediate percentage of charge (except for full discharge or full charge).
- • Optimally charge to the level 40%-60%.
- • During storage , recharge once every 3 months (otherwise self-discharge will reduce the percentage).
- • Store NiCd and NiMh for no longer than 5 years .
- • Before use after long-term storage, activate ( completely discharge and charge ).
Types of phone batteries
If you have any questions, write them in the comments or send them as a private message to us on VKontakte @NeovoltRu.
Subscribe in the group to news from the world of gadgets, learn about improving their autonomy and progress in scientific research on batteries. Connect with us on Facebook and Twitter. We also maintain a busy blog on Zen and Medium - come check it out.
Is it possible to boost the battery when capacity decreases?
With a decrease in the capacity of Ni-Mh - Ni-Cd, it is possible to restore this parameter to a significant extent. The procedure for eliminating the memory effect is carried out in the following sequence:
- Discharge the battery through a not-too-powerful consumer of electricity until a voltage of 0.8 - 1.0 Volts is present at the contacts of the product. This indicator can be measured using a multimeter.
- Place the battery in the charger and charge it to 100 percent.
- Repeat the charge-discharge process several times.
If the memory effect is the consequences of an “underdischarge” that was observed for a long time, then the charging process may need to be carried out using more powerful chargers.
If during the operation of batteries the memory effect does not clearly appear or the products are stored for a long time without recharging, then the training described above is recommended for preventive purposes. This approach works especially well when using Ni-Mh and Ni-Cd batteries.
Still have questions or have something to add? Then write to us about it in the comments, this will make the material more complete and accurate.
| Contacts | Price lists | Company news | Delivery of goods | FAQ | Avenues | Instructions | Site diagram |
Home => FAQ => NiCd, NiMh, LiIon batteries. Help in choosing a battery
All about batteries. Batteries for radios and mobile phones.
1. Something you need to know. Battery memory effect.
The memory effect of nickel-based batteries has been known for a long time. And if earlier it was understood as a kind of memory by the battery of the state in which it was before subsequent charging, now the interpretation of this term is different. The external manifestation of the effect is a decrease in the actual battery capacity during operation.
The amount of energy a battery can hold (actual capacity) gradually decreases with use and aging, and due to insufficient maintenance for some electrochemical system batteries. The battery must eventually be replaced when its capacity drops to 60-70% of its rated value. Individual Russian users, as a rule, manage to use batteries until their capacity drops to 20–30% of the nominal value. A capacity value of 80% is usually taken as the lower acceptable value for a new battery. Branded (registered) new batteries, as a rule, have a real capacity close to 90%, batteries from third-party manufacturers - often about 70%.
NiCD and, to a lesser extent, NiMH batteries are susceptible to memory effect. At the moment, the memory effect is understood as a reversible loss of capacity caused by the enlargement of crystalline formations of the active substance of the battery and thereby reducing the active surface area of its working substance.
Modern batteries
The essence of the phenomenon is that with small crystalline formations of the internal working substance of the battery, the surface area of the crystalline formations is maximum, and, consequently, the amount of energy stored by the battery is maximum. When crystalline formations become larger during operation, their surface area decreases and, as a result, the actual capacity decreases.
Is it possible to “split” these enlarged formations and return them to their original state? Yes, it is possible, if the process of their consolidation has not gone too far. To do this, it is necessary to periodically train nickel-based batteries: NiCD - approximately once a month, NiMH - once every two months. In this case, training means completely discharging the battery to a voltage of 1 volt per cell (if you, for example, have a battery with a nominal voltage of 6V, i.e., 5 cells in the battery, then it must be discharged to 5V) and then fully charging. It may take up to 3-5 such discharge/charge cycles to restore battery capacity. As a rule, the battery does not discharge directly in the phone to this voltage - the phone turns off at a higher voltage. The best effect is achieved in some chargers with a discharge function.
However, it should be noted that some of the reconditioned batteries may have high self-discharge due to crystalline damage to the separator material. This is usually characteristic of old batteries.
Ultimately, what can be advised to the average consumer using NiCD or NiMH batteries? Try to use them in the following mode: charge, use until the end, and only then charge again.
The situation is completely different with lithium-ion (Li-ION) batteries, which rather like to be in a charged state. They can be charged at any time and kept in the charger as long as you like. It is only important that the charger is designed to charge Li-ION batteries. Such chargers turn off the charging current after charging is complete. Another important feature of Li-ION batteries, as well as sealed lead-acid (SLA) batteries, is the need to store them only in a charged state.
2. A little about charging. How to properly charge the battery?
The old lady bought a car, drove it some distance, and suddenly the engine stopped. The called technical support service stated that the gas had run out. A perplexed old woman is suing: during the sale, no one explained to her that the car still needs to be filled with gasoline...
So, the batteries need to be charged. This is their significant difference from batteries. But before we talk about chargers, let's briefly look at the basic methods of charging the most common types of batteries. It should be noted that the charging methods for nickel-based batteries are different from the charging methods for lithium-ion batteries. Therefore, when charging the latter, pay attention to which charger you insert them into. In other words, not every nickel-cadmium (NiCD) and nickel-metal hydride (NiMH) battery charger is suitable for charging lithium-ion (Li-ION) batteries.
A few words about terminology. Battery capacity is usually designated by the letter C (capacity). When they talk about a discharge equal to 1/10 C, this means a discharge with a current equal to a tenth of the nominal capacity of the battery. So, for example, for a battery with a capacity of 1000 mAh this will be a discharge current of 1000/10 = 100 mA. Theoretically, a 1000 mAh battery can supply 1000 mA for one hour, 100 mA for 10 hours, or 10 mA for 100 hours. In practice, at high discharge current values the rated capacity is never reached, and at low currents it is exceeded. Similarly, when charging batteries, a value of 1/10 C means charging with a current numerically equal to a tenth of the declared battery capacity.
slow charge - charge with a constant current of 0.1 C or 0.2 C for approximately 15 or 6–8 hours, respectively. With this method, several options are possible: charging with semi-constant current and charging with constant current. When charging with semi-constant current, the initial current value is set to approximately 1/10 C. As charging continues, this value decreases. Charging time is approximately 15–16 hours. In practice, the method is implemented by charging through a current-setting resistor from a constant voltage source. A slow charge of 1/10 C is usually safe for any battery. When charging with constant current, a current value of 1/10 C is maintained throughout the entire charging time. During charging, the voltage across the battery cell increases. Upon reaching full charge and when recharging, the voltage begins to decrease. Reducing the charging time by 2–2.5 times is possible by increasing the current to 0.2 C, but in this case it is necessary to limit the charging time to 6–8 hours.
fast charge - charge with a constant current of 1/3 C for about 3-5 hours. A type of slow charge is the fast charge method, which uses a charge current of 0.3 to 1.0 C. But this can cause the battery to overheat, especially at charge currents close to 1 C. To prevent overheating and determine when the battery has finished charging, a thermal fuse is built into the battery and a temperature sensor. The temperature sensor is used to measure temperature, the change of which is considered as a criterion for stopping the charge. The fact is that when a full charge is reached, the temperature of the battery cells rises sharply. And when it rises by 10 degrees Celsius or more relative to the environment, the charge must be stopped, or switched to slow charge mode. With any charging method, if high charging currents are used, a safety timer is additionally required.
accelerated or delta V charge - a charge with an initial charge current equal to the nominal capacity of the battery, in which the voltage on the battery is constantly measured and the charge ends after the battery is fully charged. Charging time is approximately an hour and a half. This is the best and perhaps the main method for quickly charging NiCD and NiMH batteries for cell phones. The essence of the method is to measure the change in voltage on the battery to determine (fix) the moment of full charge and the need to stop it. If you measure the voltage at the battery terminals during direct current charging, you will notice that the voltage first slowly increases, and at the point of full charge it will decrease briefly. The magnitude of the decrease is small, approximately 15–30 mV per cell for NiCD and 5–10 for NiMH, but is clearly pronounced. This small drop in voltage is taken as the criterion for stopping the charge. In addition, the delta V charging method is almost always accompanied by a temperature measurement, which provides an additional criterion for assessing the state of charge of the battery (and to be sure, chargers for large high-capacity batteries usually also have safety timers).
There are electronic circuits designed specifically to implement the delta V charge method. Implementing charging using this method is more difficult and expensive than others, but gives highly reproducible results. At the same time, it should be noted that in a battery with at least one bad element from a chain connected in series, the delta V charge method may not work and lead to the destruction of the remaining elements.
NiMH batteries have specific charging problems. Their delta V value is very small and is more difficult to detect than with NiCD batteries. Therefore, NiMH cell phone batteries have temperature sensors as a backup to detect when they are fully charged.
Another problem with charging with this method is that when used in cars, electrical interference masks the delta V detection and phones mostly control charge based on temperature. This can damage the battery since the phone is always connected in the car and the engine starts and stops repeatedly. Each time the ignition is turned off for a few minutes and then turned back on, a new charge cycle is initiated.
To charge Li-ION batteries, the “constant voltage/constant current” method is used, the essence of which is to limit the voltage on the battery. In this way it is similar to the lead-acid (SLA) battery charging method. The main differences are that for Li-ION batteries there is a higher voltage per cell (nominal cell voltage 3.6V versus 2V for SLA), a tighter tolerance for this voltage (+/– 0.05V) and the absence of slow recharging after a full charge. .
A higher voltage threshold provides a higher capacitance value, so it is in the manufacturer's best interest to select the highest possible voltage threshold without compromising safety. However, this threshold is affected by the temperature of the battery and is set low enough to allow elevated temperatures during charging.
In chargers and battery analyzers that allow you to change the value of this voltage threshold, its correct setting must be observed when servicing any Li-ION battery type. However, most manufacturers do not indicate the type of Li-ION battery and the end-of-charge voltage. And, if the voltage is set incorrectly, the battery with a higher voltage will produce a lower capacity value, and the battery with a lower voltage will be slightly overcharged. At moderate temperatures there is no damage to batteries.
This is, as a rule, the reason that a battery charged, for example, in a “native” phone, lasts less or longer than the same battery charged in a desktop charger from an unknown manufacturer.
Consumer intervention with any Li-ION charger is not recommended. Slow recharging at the end of the charge, characteristic of nickel-based batteries, is not used because the Li-ION battery does not tolerate overcharging. Slow charging can cause lithium metallization and lead to cell destruction. Instead, a short-term charge can be applied from time to time to compensate for the small self-discharge of the battery due to the small current consumption of the protection device.
Li-ION batteries contain several built-in protection devices: a fuse, a thermal fuse, and an internal control circuit that turns off the battery at the low and high points of discharge and charge voltage.
Never attempt to charge lithium batteries! Attempting to charge these current sources may cause an explosion and fire, which will spread toxic substances and may cause equipment damage. If the lithium-ion battery ruptures, leaks electrolyte and gets on your skin or eyes, immediately rinse these areas with running water. If electrolyte gets into your eyes, rinse them with running water for 15 minutes and consult a doctor.
3. Chargers and battery charging technologies.
Let it charge your battery overnight? – Perhaps, soon fewer and fewer people will be asking this question. Mobile technologies are rapidly breaking into our lives and making us think about things completely unusual for us before. Chargers are also from this category. Well, tell me, why do we, ordinary users, care about all this wisdom? But no, you have to ask, figure it out, find out how and what is better. Do you or we need this? The choice is yours!
Let us now turn directly to the devices that implement them, or, simply put, to the chargers. But at the same time, we’ll immediately make one significant clarification: mobile devices are an increasingly expanding concept at the moment. And it includes both cell phones and radios, as well as ordinary home cordless phones, DECT radiotelephones, all kinds of portable and handheld computers, video cameras, digital cameras and much more. What they all have in common is that they are powered by batteries. But from batteries... - different ones! Moreover, they differ not only in the type of electrochemical system (nickel-cadmium, nickel-metal hydride, lithium-ion or lithium-polymer), but also in their electrical characteristics. Hence the consequence: chargers for each of them have their own characteristics.
So, chargers are: > according to the type of electrochemical system of rechargeable batteries - for nickel-based batteries: nickel-cadmium (NiCd) and nickel-metal hydride (NiMH), for lithium-ion (Li-ion) batteries and combined; > by design – built into the phone or into an external power supply (designed to charge the battery directly in the phone) and desktop; by charging method - devices charging with direct current and devices with a pulse charging method; > by charging time – slow and fast; > by input supply voltage – devices connected to the AC voltage network and to the vehicle’s on-board network; > by functionality – household and professional chargers;
As a rule, chargers designed to charge the battery directly in the phone cause the least problems for users. The fact is that phone manufacturers are trying to match the charging technology as much as possible with all possible types of batteries designed to work with a given brand of phone. Thus, if the phone is designed to work with NiCd, NiMH and Li-ion batteries, this means that its built-in charger will charge all of the above batteries equally effectively, even if they have different capacities. One of the disadvantages can be noted: for nickel-based batteries, periodic full discharge is recommended, but the phone cannot do this. When a certain voltage threshold is reached, it turns off. This turn-off voltage exceeds the voltage to which the battery must be discharged in order to prevent a decrease in its capacity that occurs during operation. In this regard, a desktop charger with a discharge function is preferable.
Is it possible to get by with just the phone's built-in charger? Of course it is possible, but perhaps not very convenient. To avoid unforeseen situations caused by insufficient battery charge in broad daylight, it’s a good idea to have a desktop charger at your place of work or a spare battery (although you can’t put two batteries in your phone, for example, overnight, to charge it). Its choice must be approached more carefully. Firstly, not all devices can effectively charge lithium-ion batteries. For example, the DP Battery company clearly stipulates that only chargers with the EP (Expert Performance) logo must be used to charge lithium-ion batteries. Secondly, each charger is designed to charge batteries of a certain capacity. Keep in mind that a slow charger designed to charge small capacity batteries may not be able to fully charge a higher capacity battery in the same amount of time, or even longer. And, conversely, a fast charger with a high charge current can recharge a battery with a small capacity. On the other hand, desktop chargers can have two seats: the first for the phone along with the battery and the second for only the battery. Moreover, often in the second seat the battery can be completely discharged before charging, which is periodically recommended to do for nickel-based batteries and only for them. Lithium-ion batteries do not need to be discharged before charging.
Slow and fast chargers differ in charging speed. The first ones charge the battery with a current approximately equal to 1/10 of the nominal capacity of the battery, and the charging time is 12–15 hours. Hence their other name - nocturnal. The latter charge the battery with a current in the range from 1/3 to 1 of its nominal capacity for three to one hour. Which is preferable? Whichever is more convenient for you. Fast charging of cell phone batteries does not significantly affect their service life. At least, battery manufacturers do not provide information about a decrease in service life and allow charging with such a current. In all other cases, you must consult the seller. It should be noted that you should not leave NiCd and NiMH batteries in the charger after the end of charging (overnight is possible), since the charger for these batteries after they are fully charged does not stop charging, but continues, but only with a significantly lower current. Leaving NiCd and NiMH batteries in a charger for a long time leads to their overcharging and deterioration of their parameters. This does not apply to Li-ion batteries and chargers for them. Li-ion batteries prefer to be in a charged state rather than in a discharged state. In addition, chargers, after fully charging the battery, reduce the charging current to zero and therefore Li-ion batteries can be left in the charger after charging is completed.
To extend the service life of NiCd batteries, we can recommend the use of chargers with a pulse charging method, which reduces crystalline formations in NiCd batteries (“memory effect”) that occur during operation (but unfortunately, the descriptions of chargers often do not mention any charging methods, nor about methods for monitoring the achievement of a full charge). However, for batteries with a large “memory effect”, the use of the pulsed charging method alone is not enough and therefore a deep discharge (recovery) is necessary according to a special algorithm in order to destroy more resistant crystalline formations. Conventional chargers, even with a discharge function, do not do this.
For everyone who intensively uses a car, a car charger option is certainly necessary. But only as a last resort. You should not abuse the battery charge in a car, especially while driving around the city, with frequent stops and repeated starts of the engine. Such charging conditions are unfavorable for the battery. The temperature conditions of the charge in the car also negatively affect it, especially in winter and summer. The best location for a phone with a battery in such conditions is under outer clothing in winter, and in the coolest place in summer.
In conclusion, I would especially like to dwell on charging batteries for home radiotelephones (not to be confused with radio extenders). As a rule, users constantly keep the handset on the “base”, some out of ignorance, some to protect against unauthorized connection. And since a simple slow charger for them, as a rule, is built into the “base,” the constant presence of the handset on it leads to uncontrolled charging and damage to the batteries. The fact is that cordless phones usually use nickel-cadmium batteries, which are recommended to be charged after they are fully charged. So, place the handset on the base only after it signals you to charge.
4. How to determine the current state of the battery?
As a rule, most people rarely think about the condition of their mobile phone battery, believing that it will faithfully serve us for a long time. Time passes: a month, two, three... At the subconscious level, there is a feeling that something is wrong with the mobile phone - its operating time with a fully charged battery has become shorter. What is the reason? The answer is simple - the condition of the battery has deteriorated.
The battery, like any other thing, ages, deteriorates from improper handling and needs some care, or, in the language of specialists, maintenance. There is nothing you can do about the aging of the battery, but it is within your power to take care of maintaining it in good condition.
What do you mean by good condition? In relation to a battery for a mobile (cell) phone or radio station, a good condition means that its three main parameters are within normal limits: actual electrical capacity, internal resistance and self-discharge. Electrical capacitance directly affects the operating time of the phone, both in standby mode and in talk mode. The phone's ability to work in conversation mode or receive messages directly depends on the internal resistance. Self-discharge affects the rate at which electrical capacitance decreases over time. And the question immediately arises: how can these battery parameters be checked and evaluated?
Estimation of real electrical capacitance
There are two ways to do this. The first method is qualitative and very approximate. It consists of an approximate estimate of the average operating time of your mobile phone. However, the difficulty is that this time depends on many factors: the nominal capacity of the battery, the degree and correctness of its initial charge, the frequency and duration of your telephone calls, the distance from the base station, and the ambient temperature. Therefore, a slight decrease in battery capacity (by 15–20%) usually goes unnoticed. The second method, which is more accurate, requires special chargers with a battery discharge function. In this case, it is enough to charge the battery in the charger, and then put it on discharge and estimate the time that will pass from the moment the discharge begins until the moment it ends and automatically switches to charge. Knowing the duration of this time interval for exactly the same known-good battery and determining this interval for the battery under test, you can estimate its capacity. The only inconvenience is that you have to sit in front of the charger and wait for it to finish draining the battery. This method allows you to evaluate to some extent the internal resistance of the battery if the charger provides a discharge current of 500 mA or higher.
Estimation of internal resistance
The main sign of a high internal resistance of the battery is that the telephone or radio turns off when an outgoing or incoming call starts (provided that the battery contacts are clean and there are no signs of oxidation or damage). In such cases, users usually say that they charged the battery until the “green light” in the charger or in the phone, turned it on, and after a while it turned off (the shutdown occurred at the time of an incoming call, but the call was not heard!). Or the phone turns off during an outgoing call. The battery voltage indicator (until the moment of shutdown) shows that it is still possible to work. All of the above cases are qualitative signs of a high internal resistance of the battery. It is not possible for the average user to quantify its value in everyday life.
Self-discharge rating
The essence of self-discharge is the loss of capacity by the battery in relation to the value it has immediately after charging. To evaluate it, you will also need a charger with a discharge function. Charge the battery and discharge it immediately after. Record the time interval required for the battery to be completely discharged. Charge the battery again and set it aside for a day. The next day, after 24 hours, discharge the battery in the same charger and determine how long it will take until it is completely discharged. Compare these two time intervals. Naturally, the duration of the second interval will be shorter, and this decrease approximately shows how much your battery has lost per day. Thus, you can evaluate the self-discharge of nickel-cadmium and nickel-metal hydride batteries (loss of capacity during the first day after charging should be no more than 10-15%). The self-discharge of lithium-ion batteries is estimated a month after charging due to its small value.
5. Conclusion.
We hope that this article will help you not only in choosing the right type of battery, but also in operating these devices. In conclusion, I would like to add. The condition of any battery, even the most modern, deteriorates during use and aging. The degree of degradation depends on the battery type, operating conditions, maintenance and handling. Without timely removal of weak batteries, the benefits of modern ultra-high capacity batteries are lost. Thus, batteries with high energy density are only superior to older types when their technical condition is frequently checked. Implementing a reliable battery maintenance system is essential for reliable service and to prevent unexpected device or system failure. Also regarding mobile phones. If the phone is used for business calls, weak or old batteries should not be used at all. Think for yourself, how much will a missed call due to a dead battery cost you?
m."109316 Moscow, Volgogradsky prosp., 26s1of. 1406 (1 minute walk from Volgogradsky Prospekt metro station), office)" Opening hours: from 1000 to 1800 without lunch, also almost always in the evening until 22.00 and on weekends from 15.00 to 22.00 (check by phone!). +7(903)7550303 +7(903)9680380 ,
https://www.radiovnimanie.ru, e-mail
Processes of interaction of elements in a battery
In conclusion, it is worth considering the condition of the elements in the battery. You probably know that nickel-metal hydride batteries are rarely used one cell at a time. More often they are used in a set of some kind of battery. For example, a battery for a screwdriver with an operating voltage of 14.4 volts can be composed of 10-12 individual elements connected in series.
Bosch screwdriver battery
Different elements receive a certain range of characteristics during production.
Some have more capacity, while others have less. As a result of constant charging in a bundle, elements with a lower capacity are constantly recharged. Because of this, their rapid degradation occurs. If there are short-circuited elements in the assembly, then because of this there will be a constant recharging of the rest. Batteries with a smaller capacity will also degrade when discharged. They discharge earlier than other elements. Further discharge leads to their deep discharge, and sometimes polarity reversal. Therefore, repairing a screwdriver battery is often done with a simple set of serviceable elements from the main and spare batteries.
During operation, whenever possible, you should strive to ensure that the degree of charge of individual batteries is the same. So, with periodic recovery, you can train the elements separately. Since this requires disassembling the assembly, it can be difficult. Therefore, advanced chargers are equipped with a balancing or equalization mode. It can be recommended for new and deeply discharged alkaline batteries.
When balancing, if the battery is very discharged (less than 0.8 volts), charging is carried out to a voltage of 1 volt with a current of 0.1 * C. Next, charging is carried out with a current of 0.3 * C, limited in time to 4-5 hours. It is recommended to do several charge-discharge cycles if the battery is stored for a long time before using it.