Selecting solder for soldering
The choice of solder is made depending on the following factors:
- metals or alloys to be joined
- soldering method
- temperature restrictions
- part size
- required mechanical strength
- corrosion resistance, etc.
To solder thick wires, use solder with a higher melting point than for soldering thin wires. In some cases, the conductivity of the solder must also be taken into account (reminder: the resistivity of tin is 0.115 ohm x mm2/m, and that of lead is 0.21 ohm x mm2/m).
Types of solders
Solder for soldering
Solder for soldering is divided into three groups: refractory, low-melting and ultra-low-melting.
Refractory solders (radio amateurs practically do not use them). Refractory solders include solders with a melting point above 500 °C, which create very high mechanical strength of the joint (tensile resistance up to 50 kg/mm2). Their disadvantage is precisely that they require high heating temperatures and, although the strength of such soldering is very high, intense heating can lead to undesirable consequences: you can, for example, “release” a steel part. The disadvantage of hard solders is that they require high heating temperatures, and although the strength of such soldering is very high, intense heating can lead to very undesirable consequences: you can overheat an expensive part and damage it (for example, a transistor or microcircuit), you can “let go” ", for example, a steel part (spring).
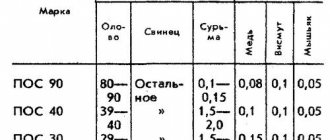
Low-melting (ham radio) solders. This category includes solders with a melting point of up to 400 °C, which have relatively low mechanical strength (tensile strength up to 7 kg/mm2). In radio engineering installation work, low-melting solders are mainly used. They contain tin and lead in various proportions, for example, POS-61 solder, which contains 61% lead, 38% tin and 1% various additives.
Ultra-low-melting (ham) solders. There are also alloys that contain, in addition to tin and lead, bismuth and cadmium. These alloys are the most fusible: some of them have a melting point of less than 100 °C. The mechanical strength of the connection of such alloys is very low. Previously, they were used for soldering crystals in crystal detectors. Currently, low-melting cadmium-bismuth alloys are used in the repair of printed circuit boards. They are also used for soldering transistors, since according to technical specifications it is recommended to solder them with solder with a melting point not exceeding 150 °C.
For soldering transistors, you can use the so-called Wood's alloy with a melting point of 75 ° C, which contains: tin - 13%, lead - 27%, bismuth - 50%, cadmium - 10%. Wood's alloy can be prepared according to the specified recipe yourself or purchased at a pharmacy. Soldering is carried out with a weakly heated soldering iron. Rosin is used as a flux.
How to use soldering flux
To correctly apply soldering flux, you need to look at its consistency:
- If hard solder is used, for example, from tin, then the soldering iron itself needs to be dipped in the reagent, and then take a little solder.
- Liquid flux assumes that it will be applied with a special brush. You need to be careful here, as brushes often quickly deteriorate due to high temperatures.
- The paste is applied with a stick, toothpick or syringe with the tip of the needle cut off.
And then act like this:
- Clean the surface from oxides. Sometimes this is not required if the flux allows it.
- A layer of flux is applied.
- The composition and parts are heated at the soldering station.
After soldering is completed, you need to wait until the seam hardens.
Amateur radio solder for soldering
Nowadays, solder wire with a cross-section of 1 to 5 mm is used for soldering. The most common are 1.5-2 mm multi-channel solders. Multi-channel means that inside the tin wire there are several channels of flux, which ensures the formation of an even, shiny and reliable solder. Such solder is sold in coils - at radio markets, in flasks - in which it is rolled into a spiral, and in reels (the amount of solder in them is such that it will last for more than one year). It is recommended to purchase it in the form of a wire, the thickness of a match - it is more convenient to solder.
When soldering installation wires of radio equipment, it is convenient to use tin-lead solders, cast in the form of thin rods with a diameter of 2 - 2.5 mm. You can make such rods yourself by pouring molten solder into a vessel with a hole pre-made in the bottom. The vessel should be held above a sheet of tin or a metal plate. After cooling, the rods should be cut into pieces of the required length.
Modern solders used in soldering electronic circuits are produced in the form of thin tubes filled with a special resin (colophonium), which acts as a flux. Heated solder creates an internal connection with metals such as copper, brass, silver, etc., if the following conditions are met: the surfaces of the parts to be soldered must be cleaned, that is, the oxide films formed over time must be removed from them, the part is in place Soldering joints must be heated to a temperature above the melting point of the solder. Certain difficulties arise in the case of large surfaces with good thermal conductivity, since the power of the soldering iron may not be enough to heat it.
What is flux?
Flux is an auxiliary material that is designed to remove the oxide film from parts being soldered during soldering and ensure good wetting of the surface of the part with liquid solder. Without flux, solder may not adhere to the metal surface. Purpose of fluxes: reliably protect the surface of the metal and solder from oxidation, improve the conditions for wetting the metal surface with molten solder. The effect of a flux depends on its composition; available fluxes either dissolve oxide films on the surface of the metal (and sometimes the metal itself), or protect the metal from oxidation when heated. Thus, the flux forms a protective film over the soldering area.
Soldering fluxes
Flux is already contained in modern solder in the form of a thin core. When solder melts, it is distributed over the surface of the liquid metal. The surfaces of already tinned metals are also coated with flux before joining them (actually soldering). In this case, flux is a surfactant, that is, a Surface Active Substance. After the parts come into contact, excess flux between them comes out and evaporates all the time because its evaporation temperature is lower than that of solder.
Fluxes are different. For example, to repair metal utensils, they use “soldering acid” - a solution of zinc in hydrochloric acid. You cannot solder radio structures with this flux - over time it destroys the soldering. For radio installation, it is necessary to use fluxes that do not contain acid, for example, rosin.
Flux brands
Flux is an auxiliary substance necessary to free the surfaces of soldered parts from oxides and promote better spreading of solder over the metal surface during soldering.
In the manufacture of the most common fluxes, rosin is used. It is obtained from coniferous wood (mainly pine). At a temperature of about 50°C, rosin softens, and at 250°C it begins to boil.
Rosin is not resistant to atmospheric moisture. It consists of 85-90% abietic acid. If you do not remove its remains after work, then oxidation of the soldering area occurs. This fact should not be ignored. Indeed, in addition, by absorbing water from the atmosphere, rosin increases its conductivity and can disrupt the operation of electronic devices, especially high-voltage circuits.
Soldering fluxes - composition and scope of application:
| Name | Composition % of total volume | Application area | Cooking method | Removing residues |
| Rosin inactive fluxes | ||||
| Light rosin | Light rosin – 100 | Soldering of copper and its alloys with low-melting solders | Ready to use | Alcohol or acetone, brush |
| Alcohol-rosin | Rosin - 20 Alcohol - 80 | Soldering of copper and its alloys with low-melting solders in hard-to-reach places | Dissolve rosin powder in ethyl alcohol | |
| Glycerin-rosin | Rosin - 6 Glycerin - 14 Alcohol - 80 | Sealed soldering of copper and its alloys with low-melting solders in hard-to-reach places | Dissolve rosin powder in ethyl alcohol, then add glycerin | |
| Rosin active fluxes | ||||
| Rosin zinc chloride | Rosin - 24 Zinc chloride - 1 Alcohol - 75 | Soldering of non-ferrous and precious metals, critical parts made of ferrous metals | Dissolve mixed powders of rosin and zinc chloride in ethyl alcohol. | Acetone, brush |
| Rosin zinc chloride (flux paste) | Rosin - 16 Zinc chloride - 4 Vaseline - 80 | High-strength soldering of non-ferrous and precious metals, critical parts made of ferrous metals | Mix rosin and zinc chloride powders with technical petroleum jelly | |
| Acidic active fluxes. | ||||
| Zinc chloride | Zinc chloride - 25 Hydrochloric acid - 1 Water - 75 | Soldering of parts made of ferrous and non-ferrous metals | The acid is slowly poured into the dish up to ¾ of its height with pieces of zinc, when the hydrogen bubbles stop emitting, the flux is ready | Washing with water or a solution of baking soda in water, with a brush |
| Rosin - 16 Zinc chloride - 4 Vaseline - 80 | Flux paste. High-strength soldering of non-ferrous and precious metals, critical parts made of ferrous metals | Mix rosin and zinc chloride powders with technical petroleum jelly | ||
| Rosin - 24 Zinc chloride - 1 Alcohol - 75 | Soldering of non-ferrous and precious metals, critical parts made of ferrous metals | Dissolve mixed powders of rosin and zinc chloride in ethyl alcohol. | ||
Flux based on alcohol and solvents must be stored in an airtight container, otherwise the liquid will quickly evaporate.
Requirements for amateur radio fluxes
The choice of flux is an important issue. Previously, only rosin was used, there was no other flux. Why rosin is bad - rosin, alcohol rosin flux belong to the category of active fluxes. The first disadvantage is that at high temperatures, not only the metal oxide is removed, but also the metal itself. The second drawback is that cleaning the board after soldering with rosin is a big problem. You can only wash off the residue with alcohol or solvents (and even then, sometimes it’s easier to pick it out with something sharp). Flux residues on the board are not only unsightly from an aesthetic point of view, but also harmful. On boards with small gaps between conductors, the growth of dendrites (in other words, short circuits) caused by galvanic processes on a contaminated surface is possible. What is the solution - on the modern materials market you can find a wide range of fluxes that are washed off with ordinary water, do not destroy the soldering iron tip and provide high quality soldering. Such fluxes are usually sold in syringes, which is very convenient for use.
Which flux is better to choose?
To choose a flux for soldering, you also need it to be suitable for the material to be soldered:
- For copper, for example, rosin is often used. It is suitable for any simple electronics with a large number of wires.
- Liquid solders with Vaseline or salicylic acid are useful for radiators and wires with one core.
- Liquid rosin is suitable for multi-core wires.
- The paste-like composition is suitable for radio components and various connectors, for SIM cards and flash drives, for example.
- Wires and connectors require active fluxes for soldering.
- For small radio components on boards, neutral fluxes in paste are suitable. When working with boards, you need to choose products that will not stain the board itself, since it is almost impossible to remove the product from the surface around the soldering area.
- Typically, activated compounds that do not require rinsing are chosen as flux for soldering microcircuits. They should be liquid or gel-like.
Also, when choosing fluxes, you should read other people’s reviews in order to make a choice from trusted brands, since many companies produce soldering products, but not all of them are of high quality.
How to replace flux
Regardless of what flux is used, the finished soldering must be wiped with a cloth soaked in rectified alcohol or acetone, and also cleaned with a stiff brush or brush moistened with a solvent to remove flux residues and dirt. In some exceptional cases, instead of rosin, you can use its substitutes:
- rosin varnish, available in hardware stores. It can be used as a liquid flux instead of a solution of rosin in alcohol. The same varnish can also be used for anti-corrosion coating of metals.
- resin - pine or spruce resin - is an accessible material, especially for amateurs living in rural areas. You can prepare this flux yourself. Resin collected from trees in the forest must be melted in a tin over low heat (the resin may ignite over high heat). Pour the melted mass into matchboxes.
- an aspirin tablet available in any home medicine cabinet. The disadvantage of this flux is the unpleasant smell of smoke released when aspirin melts.
Nowadays a large number of different so-called “no-clean” fluxes are produced, both liquid and in the form of a semi-liquid gel. Their peculiarity is that they do not contain components that cause oxidation and corrosion of the parts being connected, do not conduct electric current and do not require washing the board after soldering. Although it is still better to remove all flux residues from the soldered parts after completing soldering.
To apply liquid flux, you can use a brush, a cotton swab or just a match, but it is more convenient to use the so-called “flux applicator”. You can try to buy a branded flux applicator that costs about $20-30. It is also convenient to use flux in the form of a gel or paste. To apply it, you can use a disposable syringe, but because of its thickness, you will have to use a thicker syringe needle.
Solders and fluxes. Types, characteristics, properties of solders and fluxes.
Table 2. Physical and mechanical properties of soft and semi-hard solders
| Solder grade | Melting point, °C | Density, kg/m3 | Electrical conductivity, % conductivity of copper | Tensile strength, MPa | Approximate soldering temperature, °C | |
| Solidus | Liquidus | |||||
| O2 | 232 | 232 | 7310 | 13,9 | 25 | 280 |
| POS61 | 183 | 190 | 8500 | 12,6 | 43 | 240 |
| POS40 | 183 | 238 | 9300 | 11,1 | 38 | 290 |
| POS10 | 268 | 299 | 10800 | 8,8 | 32 | 350 |
| POS61M | 268 | 192 | 8500 | 12,8 | 45 | 240 |
| POSK50-18 | 183 | 145 | 8800 | 13,2 | 40 | 185 |
| POSSu61-0.5 | 142 | 189 | 8500 | 12,6 | 45 | 240 |
| POSSu40-0.5 | 183 | 235 | 9300 | 10,4 | 40 | 285 |
| POSSu30-0.5 | 183 | 255 | 9700 | 9,8 | 36 | 306 |
| POSSu18-0.5 | 183 | 277 | 10200 | 8,9 | 36 | 325 |
| POSSu95-5 | 234 | 240 | 7300 | 12,1 | 40 | 290 |
| PSr3Kd | 300 | 325 | 8700 | 22,4 | 54 | 360 |
| PSr2.5 | 295 | 305 | 11000 | 8,8 | — | 355 |
| POSI30 | 117 | 200 | 8420 | — | — | 250 |
| PSR3I | 141 | 141 | 7360 | — | — | 190 |
Table 3. Predominant applications of soft and semi-hard solders
| Solder grade | Application area |
| O2 | Tinning and soldering of commutators, armature sections and windings of electrical machines with class H insulation, tinning of critical fixed contacts, including those containing zinc |
| POS61; POSSu61-0.5; POS61M | Hot tinning and soldering of copper and its alloys, kovar silver, nickel and its alloys. Soldering of live parts of electrical machines and devices operating at temperatures up to 160 °C |
| POS40; POSSu40-0.5 | Hot tinning and soldering of copper and its alloys, steels and various metals coated with tin, silver, nickel. Soldering of collector bands and anchor sections of most types of electrical machines, as well as devices in contact with sea water |
| POSSu30-0.5 | Hot tinning and soldering of copper and its alloys, iron, carbon and stainless steels. Tinning and soldering of wires, cables, bands, various parts of equipment and devices operating at temperatures up to 160 °C |
| POSK50-18 | Soldering of parts made of copper and its alloys that are sensitive to overheating, including soldering of copper-clad aluminum. Soldering of ceramics, glass and plastics metallized with tin, silver, nickel |
| POS10; POSSu18-0.5 | Tinning and soldering of contact surfaces of electrical devices, instruments, relays and other parts of less critical use in mass production |
| POSSu95-5; PSr3Kd | Hot tinning and soldering of collectors, anchor sections, bandages and current-carrying connections of heat-resistant electric machines with high rotation speeds; soldering of pipelines and various electrical equipment parts |
| POSI30; PSR3I | Soldering of copper and its alloys and other metals, non-metallic materials and glass with metal coatings. Soldering of electronic equipment parts. They have high fluidity and provide good adhesion of soldered surfaces |
| Note. Antimony solders are not recommended for soldering zinc and galvanized parts. | |
Table 4. Soft solders (alloys) with low melting points
| Alloy name | Chemical composition, % | Melting temperature, °C | ||||||
| Tin | Lead | Cadmium | Bismuth | Silver | Indium | Solidus | Liquidus | |
| Wooda | 12—13 | 24,5—25,6 | 12—13 | 49—51 | — | — | 66 | 70 |
| Rose | 24,5—25,5 | 24,5—25,6 | — | 49—51 | — | — | 90 | 92 |
| D'Arce | 9,6 | 45,1 | — | 45,3 | — | — | — | 79 |
| Lipovica with indium | 11,8 | 22,2 | 8,5 | 42 | — | 15,5 | — | 48 |
| Note. They are used in radio circuits with semiconductor devices and in circuits where solder is used as a temperature fuse. | ||||||||
Table 5. Chemical composition and physical and mechanical properties of solid silver and copper-phosphorus solders
| Solder grade | Chemical composition, % | Density, kg/m3 | Crystallization temperature, °C | Tensile strength, MPa | ||||
| Silver | Copper | Zinc | Phosphorus | Start | end | |||
| PSr72 | 72±0,5 | 28±0,5 | — | — | 9900 | 779 | 779 | — |
| PSr50 | 50±0,5 | 50±0,5 | — | — | 9300 | 850 | 779 | — |
| PSr45 | 45±0,5 | 30±0,5 | 25+1, –1,5 | — | 9100 | 660 | 725 | 300 |
| PSr25 | 25±0,3 | 40±1 | 35±2,5 | — | 8700 | 775 | 745 | 280 |
| PSr71 | 71±0,5 | 28±0,7 | — | 1±0,2 | 9800 | 795 | 750 | — |
| PSr25f | 25±0,5 | 70±1 | — | 5±0,5 | 8500 | 710 | 650 | — |
| PSr15 | 15±0,5 | 80,2±1 | — | 4,8+0,2, –0,3 | 8300 | 810 | 635 | — |
| PMF7 (MF3) | — | Rest | — | 7—8,5 | — | 860 | 710 | — |
Table 6. Some copper-zinc and copper-nickel brazes
| Solder grade | Chemical composition, % | Physical properties | |||||||||
| Copper | Nickel | Iron | Silicon | Bor | Zinc | Tin | Crystallization temperature, °C, + | Density, kg/m3 | Tensile strength, MPa | ||
| Solidus | Liquidus | ||||||||||
| L63 | 62—65 | — | — | — | — | Rest | — | 900 | 905 | 8500 | 310 |
| LOK59-0.1-0.3 | 60,5—63,5 | — | — | 0,2—0,4 | — | Rest | 0,7—1,1 | 890 | 905 | 8200 | — |
| PZHL500 | Rest | 27—30 | 41,5 | 1,5—2 | 0,2 | — | — | 1080 | 1120 | 8630 | 600 |
Table 7. Silver solders with lower melting points
| Solder grade | Chemical composition, % | Density, kg/m3 | Crystallization temperature, °C | ||||||
| Silver | Copper | Zinc | Cadmium | Tin | Nickel | Start | end | ||
| PSr50Kd | 50±0,5 | 16±1 | 16±2 | 18±1 | — | — | 9300 | 650 | 635 |
| PSr40 | 40±1 | 16,7+0,7, –0,4 | 17+0,8, –0,4 | 26+0,5, –1 | — | 0,3±0,2 | 8400 | 605 | 595 |
| PSr62 | 62±0,5 | 28±1 | — | — | 10±1,5 | — | 9700 | 700 | 660 |
Table 8. Preferred applications of brazing alloys
| Solder grade | Application area |
| PSr72; PSr50 | Soldering of metal-ceramic contacts and various critical current-carrying connections subject to bending and impact loads |
| PSr45 | Soldering of copper and its alloys, stainless and structural steels. Soldering of short-circuited rotor windings and damper windings of highly loaded electrical machines. Solder provides high density and strength of soldered seams |
| PSr25 | Soldering of copper and its alloys, stainless and structural steels, replaces PSr45 solder when making less critical connections |
| PSr71 | Soldering parts is similar to PSr72 solder, but where greater fluidity is required |
| PSr25f; PSr15; PMF7 | Soldering of copper and its alloys, including various current-carrying parts of machines and devices that do not experience shock and bending loads |
| L63; LOK59-0.1-0.3 | Soldering copper and cast iron. Soldered joints have high strength and perform well under shock and bending loads |
| PZHL500 | Soldering of connections operating at temperatures up to 600 °C |
Table 9. Copper-phosphorus
| Solder grade | Chemical composition, % | Melting point, °C | |
| Copper | Phosphorus | ||
| PFM-1 | 90—91,5 | 8,5—10 | 725—850 |
| PFM-2 | 92,5 | 7,5 | 710—715 |
| PFM-3 | 91,5—93 | 7—8,5 | 725—860 |
| Note. For copper-phosphorus and silver solders, borax is used as a flux in powder form or mixed with table salt. | |||
Table 10. Solders for soldering aluminum according to the electrical engineering standard OAA.614.017—67
| Solder grade | Chemical composition, % | Temperature of complete melting, °C | Soldering temperature, °C | Density, kg/m3 | ||||
| Tin O1 | Zinc | Cadmium | Aluminum A7 | Copper M0 | ||||
| P250A | 79—81 | 19—21 | — | — | 0,15 | 250 | 300 | 7300 |
| P300A | — | 50—61 | 39—41 | — | 0,045 | 310 | 360 | 7730 |
| P300B | — | 80 | — | 8 | 0,5 | 410 | 700—750 | — |
Table 11. Preferred applications of solders for aluminum soldering
| Solder grade | Application area |
| P250A | Tinning of the ends of aluminum wires, as well as dip soldering of aluminum wires with aluminum and copper tips |
| P300A | The same, soldering of connections with increased corrosion resistance |
| P300B | Fill soldering of aluminum wires with aluminum and copper parts |
Table 12. Solder for soldering aluminum (VTU Tsvetmetobrabotki 1989–56)
| Brand | Compound, % | Melting point, °C | Application | |||
| Tin | Zinc | Copper | Aluminum | |||
| A | 40 | 58—58,5 | 2—1,5 | — | 400—425 | For tinning and soldering of cable sheaths and cores |
| TsO-12 | 12 | 88 | — | — | 500—550 | For soldering wire and cable cores |
| TsA-15 | — | 85 | — | 15 | 550—600 | |
Table 13. Solder for soldering aluminum
| Solder grade | Chemical composition, % | Melting point, °C | |||||
| Aluminum | Copper | Tin | Zinc | Cadmium | Silicon | ||
| Cadmium | — | — | 36 | 40 | 24 | — | — |
| AVIA-1 | — | — | 55 | 25 | 20 | — | 20 |
| AVIA-2 | 15 | — | 40 | 25 | 20 | — | 250 |
| VPT-4 | 55 | — | — | 40 | — | 5 | 410 |
| 34-A | 66 | 28 | — | — | — | 6 | 545 |
Table 14. Fluxes for soldering with soft and semi-hard solders (electrical standards OAA.614.017-67 and OAA.614.028-68)
| Brand | Purpose | Basic data of fluxes | Cleaning after soldering | |
| Component names | Compound, % | |||
| TO | Tinning and soldering of current-carrying parts made of copper and its alloys | Pine rosin | 100 | Not required |
| KSP | Tinning and soldering of current-carrying parts made of copper and its alloys | Pine rosin | 25 | Not required |
| Technical ethyl alcohol grade B | 75 | |||
| FPP | Tinning and soldering of current-carrying parts made of copper and its alloys | Polyester resin grade PA9 | 20—30 | Not required |
| Methyl ethyl ketone or ethyl acetate | 80—70 | |||
| STUZO-12224-61 | Tinning and soldering of parts made of copper, nickel and their alloys and parts coated with copper, tin, cadmium, silver and zinc | Pine rosin | 20—35 | Swab or brush soaked in solvent or alcohol |
| Diethylamine hydrochloride | 3—5 | |||
| Triethanolamine | 1—2 | Swab or brush soaked in solvent or alcohol | ||
| Technical ethyl alcohol grade B | 76—68 | |||
| F59A OAA.614.017—67 | Tinning and soldering of aluminum and AMts alloy with each other and with copper and its alloys | Cadmium borofluoride | 10 | Running hot water or alcohol |
| Zinc borofluoride | 3 | |||
| Ammonium borofluoride | 5 | |||
| Triethanolamine | 82 | |||
| 34A OAA.614.017—67 | Soldering of aluminum and its alloys (melting point 420 °C | Cadmium fluoride | 50±6 | Hot, then cold running water |
| Lithium chloride | 32±6 | |||
| Zinc chloride | 8±2 | |||
| Sodium fluoride | 10±1 | |||
| LM1 | Tinning and soldering of iron-nickel alloys and stainless steels | Pine rosin | 20—35 | Swab or brush soaked in solvent or alcohol |
| Diethylamine hydrochloride | 3—5 | |||
| Triethanolamine | 1—2 | Swab or brush soaked in solvent or alcohol | ||
| Technical alcohol grade B | 76—78 | |||
| F38N | Tinning and soldering nichrome to each other and to copper | Diethylamine hydrochloride | 25—30 | Hot water or a brush dipped in alcohol |
| Ethylene glycol | 60—50 | |||
| Phosphoric acid | 29—25 | |||
Table 15. Fluxes for soldering copper and its alloys
| Brand | Compound, % | ||||
| Rosin | Ethanol | Triethanolamine | Diethylamine hydrochloride | Salicylic acid | |
| FKSp | 10—60 | 90—40 | — | — | — |
| FCTS | 15—30 | 81—65 | 1—1,5 | — | 3—3,5 |
| KSp | 50 | 50 | — | — | — |
| LTI-120 | 20—25 | 70—68 | 1—2 | 3—5 | — |
When soldering copper conductors, as well as grounding conductors to the armor and lead sheath of cables, use a solder paste consisting of the following components (in parts by mass): rosin - 10, animal fat - 3, ammonium chloride - 2, zinc chloride - 1, water or ethyl alcohol (rectified) - 1. Solder paste is often used as a flux according to the following recipe: rosin - 2.5%, lard - 5%, zinc chloride - 20%, ammonium chloride - 2%, technical petroleum jelly - 65.5% , distilled water - 5%.
Table 16. Fluxes for soldering and welding aluminum
| Brand | Compound, % | Melting point, °C | Application | |||||
| Potassium chloride | Sodium chloride | Lithium chloride | Sodium fluoride | Cryolite grade K-1 | Magnesium chloride | |||
| YOU | 50—55 | 30—35 | — | — | 10—20 | — | 630 | For terminating wires and cables |
| AF-4A | 50 | 28 | 14 | 8 | — | — | About 600 | Only for connecting cable cores in couplings |
| HP | 50 | — | 30 | — | — | 20 | — | |