A person can live from four to six weeks without food, but no more than three days without water.
However, not only humans, all living things need water. However, it was man who went the furthest, because people need water not only to maintain life, prepare food and hygiene, but also for much more. We use water both at home and at work. And now humanity is seriously thinking about extracting energy from water!
Of course, people have long been able to produce energy using water, for which a huge number of hydroelectric power stations built around the world are used. However, is it possible to extract energy directly from water?
How to ensure electrical safety in the bathroom
In order to protect yourself and your family members from electric shock when visiting the bathroom, you need to do everything correctly from the beginning. First of all, all electrical appliances and metal accessories located within the bathroom must be grounded - the grounding wire of each item must be connected to a common busbar, from which the common cable will go to the grounding busbar located in the distribution panel on the landing.
In addition, all household electrical appliances in the house that have a high degree of energy consumption, such as: boiler, air conditioner, jacuzzi, electric boiler, washing machine, heated electric floors, must be connected directly to the distribution panel, but only through a protective connection device (RCD), which automatically turns off the electrical supply when its leakage exceeds the permissible norm - 30 mA, but it is better to focus on an even lower value by programming the device to operate at a threshold of 10 mA.
It is also advisable to additionally insure yourself by purchasing an indispensable device in our difficult times, designed to equalize potentials, in common parlance SUP. His idea of work is simple, like everything ingenious: if the electrical circuit of the entire house or entrance has a direct connection, without breaks, the electrical potential of the circuit is the same. In the event that one of the neighbors decides to illegally save money by throwing a zero on the water pipes, a break appears in the circuit, and the potential difference can cause voltage to appear in the running water and the surface of the tap.
Requirements for material and wiring cross-section
According to electrical safety requirements for bathrooms, for wiring, it is necessary to use two-core copper wires with a cross-section of at least 2.5 mm
It is worth drawing your attention to the fact that in the case of connecting all metal and electrical appliances to a single EMS bus, it is necessary to avoid connecting two different metals, for example aluminum and copper, between which oxidation and corrosion processes can occur
In conclusion, I would like to say only one thing: if you are not a professional electrician, entrust the connection process to professionals. On the one hand, this will slightly increase the budget, but on the other hand, electrical wiring laid and connected correctly will serve you for decades and certainly will not cause such troubles as electric shock.
Water as a substance
The water molecule, as we know, consists of one oxygen atom and two hydrogen atoms. Its formula is written as follows: H 2 O. This substance can have three states: solid - in the form of ice, gaseous - in the form of steam, and liquid - as a substance without color, taste or smell. By the way, this is the only substance on the planet that can exist in all three states simultaneously under natural conditions. For example: at the Earth's poles there is ice, in the oceans there is water, and evaporation under the sun's rays is steam. In this sense, water is anomalous.
Water is also the most abundant substance on our planet. It covers the surface of planet Earth by almost seventy percent - these are oceans, numerous rivers with lakes, and glaciers. Most of the water on the planet is salty. It is unsuitable for drinking and for farming. Fresh water makes up only two and a half percent of the total amount of water on the planet.
Water is a very strong and high-quality solvent. Thanks to this, chemical reactions in water occur at tremendous speed. This same property affects the metabolism in the human body. It is a well-known fact that the adult body is seventy percent water. In a child this percentage is even higher. By old age, this figure drops from seventy to sixty percent. By the way, this feature of water clearly demonstrates that it is the basis of human life. The more water in the body, the healthier, more active and younger it is. That’s why scientists and doctors from all countries tirelessly insist that you need to drink a lot. It is water in its pure form, and not substitutes in the form of tea, coffee or other drinks.
Water shapes the climate on the planet, and this is not an exaggeration. Warm ocean currents warm entire continents. This happens due to the fact that water absorbs a lot of solar heat, and then releases it when it begins to cool. This is how it regulates the temperature on the planet. Many scientists say that the Earth would have cooled down and turned into stone long ago if it were not for the presence of so much water on the green planet.
What you need to know about grounding
If the water from the tap begins to emit current, then you should make sure that the apartment has grounding for appliances and plumbing. But in order to do grounding correctly, you need to understand how the power supply systems in apartment buildings and private buildings are arranged.
Old-style houses are equipped with a TN-C grounding system, that is, the following conductors are supplied to the object:
- Zero is a blue wire, which is used together with the phase wire (red) so that the voltage is 220 V;
- Neutral conductor PE of a protective nature (yellow-green). A special wire used directly for grounding;
- Phase wire.
Since in city houses it is impossible to drive a metal pin into the ground that serves as grounding, the following solution is used - the zero (earth) is combined with the working zero. So, there are 3 wires in the power sockets, 2 of which are combined in the distribution cabinet.
Newly built houses have a TN-CS power supply system. This system is equipped with a separate protective and working neutral conductor with re-grounding. In houses with such connections, the PUE provides protection against electric shocks, so the question “why is there electric shock in the bathroom” arises extremely rarely. The supply cable for such protection has 5 cores.
As for private houses, there are no difficulties with arranging grounding - this is done anywhere on the site. Here the owner will need to connect the ground electrode to the PE protective conductor, which is connected to the water distribution device. This serves as reliable protection against electric shock from the tap.
The faucet is electrocuted - how to find the problem
You can verify that the faucet is emitting current not only by touching it with your own hand, but also by touching the electrocuted faucet with an indicator screwdriver.
The reasons why the faucet is electrocuted may be:
1. A faulty water heater in which the power cord is in contact with the housing. Also in this case, very often the cause may be a failed water heater heating element.
You can read more about this in the previous article about what to do if your water heater is electrocuted.
2. The faucet can produce electric current and water from it even when someone nearby in the apartment has grounded electrical appliances in the apartment to batteries or water pipes.
In this case, it is better not to rush to sort things out, since in any case you will not achieve the truth. It is better to call the housing and communal services department and call an electrician in this case.
Also, your own grounding of the water heater and other electrical appliances in the apartment can help.
3. If the apartment already has a grounding bus, then maybe somehow electricity gets to it. This can happen when an electrical wire comes into contact with a ground loop.
And even if this is most unlikely, it is still not worth excluding grounding contact with a 220 Volt network. It is much easier to verify this and then cross out this problem as not valid than to suffer from an electrocuted faucet or water in the bathtub.
Causes of current leakage in multi-storey buildings
There are also cases when the entire metal frame, fittings, beams and channels of an apartment building are under tension. In this case, the water from the tap will be electric, the bathtub and heating radiators will be electric, that is, everything that conducts electricity will be electric.
The problem in this case lies in the contact of a damaged cable with electricity (for example, in the basement of a house) with the metal parts that support it. Therefore, it is necessary, first of all, to carry out an inspection and subsequent replacement of damaged electrical wires, after which the problem will be solved.
It also happens that the washing machine receives an electric shock due to the fact that it is not grounded. This current is stray; it is transmitted to all metal products in the bathroom and even to the kitchen. Therefore, the reasons why the faucet is electrocuted are also possible.
Be that as it may, the main thing is to first find the problem, and then solve it on your own, or by calling classified specialists for this.
Paints and varnishes - production
N. I. Gamayunov, V. M. Koshkin
(Kalinin Polytechnic Institute)
To study the structure and dielectric properties of sorbed water, various physical and physicochemical methods are used, in particular the dielectric method. Its essence lies in measuring the macroscopic characteristics of the polarization of a dielectric in an external electric field. In a constant electric field, the polarization of the dielectric is characterized by the static dielectric constant es, in an alternating field - by the complex dielectric constant є = є'-ie". Establishing a connection between the experimentally determined characteristics є5, є', є" and the molecular parameters of the dielectric is the main task of the theory of dielectric polarization [639, 640].
When studying sorbent-sorbate mixtures, the dielectric method is most effective when using polar sorbates, in particular water. In this case, the method consists mainly in obtaining and analyzing dielectric sorption isotherms, expressing the dependence of dielectric characteristics on the sorption value a
and frequency-temperature dependences е' and є" [641-645].
Despite the wide range of materials used in various works - sorbents, which differ significantly in structure and physicochemical properties, it is possible to note the general, most typical phenomena detected during the sorption of water. Thus, dielectric isotherms, depending on the slope Yr
/da ,
as a rule, can be divided into several sections. Each corresponds to a specific polarization process characteristic of a given moisture range of the material. It is obvious that polarization and dielectric constant
Rice. 15.1. The main types of dielectrons are symmetrical isotherms of water sorption.
See text for explanations.
Systems and their changes as the material is moistened depend on the mechanism of binding water to the surface.
One of the most common models used to explain the dielectric properties of water adsorbed on the surface is the layered model [429, 646]. According to this model, the entire water film can be conventionally represented as three layers. The first layer contains water molecules directly associated with the surface, the third, most distant layer is normal water. These layers are separated by a second, intermediate layer. In [646], an additional layer is identified that corresponds to the initial adsorption of molecules that neutralize the anomalous fields of defects. It is obvious that each layer is inhomogeneous in its dielectric properties. This heterogeneity manifests itself to the greatest extent in the region of low sorption values. For this humidification region, the three (or four) layer model discussed above loses its meaning. In this case, the use of a layered model involves the introduction of even “thinner” layers containing significantly fewer sorbed molecules. The change in mobility and orientational polarization of molecules as the sorbed layers increase determines the nature of the dielectric isotherm.
In Fig. Figure 15.1 shows different types of isotherms (curves 1-4). One of the most typical is S-shaped (Fig. 15.1, curve 2)
dielectric isotherm obtained for a number of organic and inorganic sorbents.
This isotherm consists of three sections A, B, C.
According to the layered model, the molecules of the first layer (section
A)
have a relatively low orientation ability in the electric field due to their sorption on the most active centers.
Such centers are functional groups capable of forming hydrogen bonds, crystal structure defects, and coordinatively unsaturated atoms [647]. The molecules of the second layer are more mobile and make a greater contribution to the orientational polarization of the sorbate, which is expressed in higher values of dz ' da (
section B
.
However, at sufficiently large sorption values, with the development of a network of hydrogen bonds, “cementation” of the sorbate occurs, its structure becomes more rigid, which complicates the orientation of dipoles in an electric field.
As a result, the slope d &'/da
decreases (section
C).
The sorption intervals corresponding to sections
A and B
depend on the structure and properties of the sorbent.
For example, during the sorption of water on a-Fe2O3, area A
corresponds to the formation of the first physically adsorbed monolayer, area
B
corresponds to the second, and area
C
corresponds to the third layer [648]. In the general case, however, the humidity range of each area does not have to be a multiple of the monomolecular sorption value.
The layered sorption model has limited application. It appears to be acceptable for some non-swelling minerals. For many sorbents, sorption should be considered as the process of dissolving one substance in another [84, 649]. The use in this case of sorption data, calculated, as a rule, using the BET method, is of interest in order to take into account the different sorption properties of materials when comparing and analyzing the dielectric isotherms obtained for them. The coincidence of the monosorption value determined by BET with the critical hydration value of ao (see Fig. 15.1) observed for such materials [648, 650–656] apparently should not be interpreted using the layered model. This coincidence only indicates that with a change in the nature of sorption, the dielectric properties of the sorbent-sorbate system also change. It is assumed, however, that for a<a0
Sorption occurs directly on the active centers of the sorbent, and when
a>a0
- on previously sorbed water molecules [651, 652, 655].
In [657], the change in the slope D &'/da
in areas
A
and
B
Explained on the basis of the hypothesis of a bidisperse pore structure. In accordance with this hypothesis, isotherm 3 (see Fig. 15.1) is divided into three sections. In the first sorption section, pores of the first type are filled, and in the third, pores of the second type are filled. In the second, intermediate section, pores of both types are filled. Such a sorption mechanism is possible, but it should be recognized that the applicability of the proposed hypothesis to a wider range of sorbents is unlikely, since it is necessary to take into account the distribution and type of active centers inside the pores and the nature of the interaction of molecules with them.
Not all dielectric isotherms obtained for the sorbents under study are S-shaped. In some cases, there is a lack of section A
or
C.
The interpretation of such isotherms is similar to the explanation discussed above for the corresponding sections of the S-shaped isotherm. The reasons for the absence of these areas may be not only certain sorption properties of the materials under study, but also the experimental difficulties that arise when measuring in the region of low or, conversely, high moisture contents of materials. In the latter case, difficulties in determining dielectric characteristics are associated with an increase in the electrical conductivity of the sorbent when it is moistened. It must be borne in mind that at high moisture contents of the material, a decrease in d'/da is associated with an increase in the volume fraction of free water. From the point of view of the three-layer model, the polarization of the entire film is determined practically by the polarization of the third layer, and a further increase in the film thickness has practically no effect on its dielectric constant [658].
An important role when taking dielectric isotherms is played by the accuracy of dielectric measurements. Thus, increasing the accuracy of measuring the increment in the electrical capacity of the capacitor AC as the sample in it is moistened made it possible to carry out a more detailed analysis of the dielectric isotherms of water sorption on proteins than was done previously [659]. The obtained dependences А С(а)
have the form of broken lines (Fig. 15.1, curve 4
, with
Based on NMR data, it can be assumed that the first section of the dielectric isotherm corresponds to the sorption of molecules on two active centers using two hydrogen bonds, the second, with a large slope of A
C/a,
corresponds to the sorption of molecules using one
H
-bond, and the third section, with an even greater value of A
C/a
is the sorption of water molecules on previously sorbed molecules.
According to work [655], the dielectric isotherm of water sorption on peat is also a broken line. Based on calorimetric sorption experiments, it was suggested that the first two sections of the isotherm correspond to different binding energies of molecules with sorption centers, and the third, with the highest derivative de '/da ,
— formation of hydrogen bonds between sorbed molecules during the sorption process.
It is significant that at a critical value of sorption a0
a sharp increase in the dielectric loss coefficient
r
, apparently due to a significant increase in the electrical conductivity of the material due to the formation of chains of sorbed molecules and functional groups of the sorbent - carboxyl (COOH), hydroxyl (OH) and other polar groups.
At the same time, the possibility of a relay mechanism of proton transfer along the chains was assumed, which causes a significant increase in є' and &". The presence of proton conductivity and proton polarization makes it possible to explain not only the large values of de '/da ,
but also the frequency dependence of the critical hydration a0 discovered for a number of sorbents [646, 648].
It should be noted here that when measuring dielectric characteristics, weak electric fields are used, which cannot affect the process of water sorption on hydrophilic materials. Therefore, the frequency dependence of the value of a0
indicates different mechanisms of the polarization process in electric fields of different frequencies.
Based on modern studies of the H-bond, it can be assumed that the polarization process significantly depends on the movement and position of the H-bridge proton in the electric field. Thus, in works [206, 660], when considering the influence of the medium on the structural form of a hydrogen bond complex (HBC), the dependence of this form on the dielectric constant of the medium is noted. When studying the O–H–N hydrogen bond, it was found that with an increase in the dielectric constant of the solution, a transition of the CVS from the molecular form to the ionic form occurs, followed by dissociation of the complex at higher values of r'
solution [660, 661]. It is important that the transfer of a proton along the H-bond in the HBC, as established in [662], is caused by a reorganization of the medium. Although the influence of the medium on the O–H–0 bond has been little studied, the high mobility of protons in the structure of ice still gives reason to assume that migration of the H-bond proton is possible in the CBCs formed at certain sorption values.
In Fig. Figure 15.2 shows the potential curves of the H-bridge proton in the absence (solid curves) and presence (dashed curves) of an electric field in the direction of coordinate z. Proton transfer can occur in the presence of either two or one potential minimum for the proton. In the first case, reorganization of the environment can cause a change in depth and a shift of both minima; in the second, a single minimum. In both cases, the formation of an ion pair with a higher dipole moment is determined by the rate of reorganization of the medium. For this reason, as noted by N.D. Sokolov [206], the assumption of tunneling proton transfer between two wells, as is often postulated for such systems, is hardly legitimate.
The mechanism of reorganization of the dielectric medium, which determines the transfer of protons to the CBC, is currently largely unclear. It can be assumed that an important role in proton transfer is played by the reactive field arising as a result of polarization of the surrounding medium by the dipole. In turn, the reactive field affects the dipole due to which it arose [639].
The energy of reactive interaction of one mole of polar molecules of a liquid with the surrounding isotropic medium satisfies the equation [663]:
4 (8,-1) (800+2) r", ER - T * NA
—————- ^^————————— — . (15.1)
Where LGd is Avogadro's number; — dipole moment of a molecule in vacuum; vm is the volume occupied by one molecule in an isotropic medium; — high-frequency limit 8′.
The role of the reactive field is not always taken into account, although, as shown by M. I. Shakhparonov, its contribution to the energy of intermolecular interaction can be significant. Thus, for water, relation (15.1) gives ER = -18 kJ/mol, which is about 40% of the total potential energy of intermolecular interaction in water [664]. It is important that ER
depends not only on the static dielectric constant
Es ,
which takes on large values for polar liquids, but also on Eoo. Therefore, the contribution of the reactive field to the energy of intermolecular interaction can be significant for weakly polar liquids.
In the sorbent-sorbed water system, the reactive field increases as the sorbent is moistened, which causes an increase in the dipole moment of the complex even in the case when additionally sorbed molecules do not directly interact with the complex. In this case, the change in r'
can occur not only due to an increase
in Es ,
but also due to an increase in Єоо.
This should manifest itself to the greatest extent when the increments of De5 and Deoo as a result of moistening the material differ slightly. In this case, the increase in r' of
the system is due to proton polarization to a greater extent than orientational one.
It can be assumed that when a weak electric field is turned on when measuring the dielectric characteristics of the sorbent-sorbate system, dipole orientation occurs, which promotes proton transfer along the H-bond. The latter causes the transition of VBC from molecular to ionic form. The probability of such a transition in the sorbent-sorbate system depends on the dielectric constant of the medium surrounding the SBC; it increases sharply at a critical value a0 determined for a given system.
In turn, the dielectric constant є' of the system increases with a decrease in the frequency of the electric field and an increase in the humidity of the material. Therefore, on dielectric sorption isotherms taken at high frequencies of the electric field, the critical value a0
is achieved at higher moisture contents of the material compared to isotherms taken at low frequencies.
The minimum value of a0
corresponds to measurements in a constant electric field.
As noted in [646], this value of a0,
according to the layered sorption model, corresponds to the completion of filling the monolayer.
It should be noted that an increase in proton polarization due to an increase in the length of chains of sorbed molecules and functional groups of the sorbent during sorption can occur if the formation of such chains increases the probability or distance of the proton jumping the H-bridge when the electric field is turned on. At the same time, for sorbents with a frequency dependence of a0, the surrounding molecules and polar functional groups play a special role in proton transfer. The orientation of their dipole moments and changes in the position of individual ions can significantly affect the characteristics of the hydrogen bond and the dynamics of the movement of the H-bridge proton [665].
Thus, analysis of dielectric isotherms of water sorption on hydrophilic materials in the region of small sorption values shows that the dependences є' = /(a) observed for various materials can be explained using two main types of polarization: orientational, due to the orientation of sorbed molecules and polar groups sorbate, and proton, associated with a change in the position of the H-bridge proton. In this case, the nature of the dependence E ' =
F ( A
) is determined by the change in the mobility of sorbed molecules and protons during the sorption process.
It should be noted, however, that the interpretation of dielectric isotherms is currently of a qualitative nature, and the dielectric method does not provide direct evidence of the existence or predominance of certain types of polarization. In this regard, the question arises of taking into account the polarization caused by the detachment (dissociation) of ions from functional groups or from the surface of the crystal lattice as polar groups of molecules are absorbed and move in associates or films of the sorbed liquid under the influence of an electric field. The accumulation of ions at the interface between different phases or components of a mixture when an electric field is turned on leads to Maxwell–Wagner polarization [666, 667], which decreases with increasing frequency of the electric field. Therefore, when measuring dielectric characteristics at high frequencies, the role of this effect is insignificant. Another way to reduce Maxwell-Wagner polarization is to conduct experiments at low temperatures [668].
Currently, it is difficult to determine the moisture content of a material, starting from which the magnitude of this polarization is comparable with other types of polarization. The studies carried out in [669] on the processes of relaxation polarization of moistened crystals characterized by high solubility give reason to believe that the role of dissolved ions in the polarization of the sorbent-sorbate mixture in the region of small sorption values is insignificant. Despite the relatively large value of є', it was suggested in [648] that there is no ionic polarization if at least three layers of adsorbate are formed.
Studies of the “non-solvent volume” [1, 670] indicate an increasing role of ions in the polarization of the sorbent-sorbate mixture at high moisture contents of the material. However, these studies do not yet allow us to judge the ion polarization of the material at low moisture contents.
The influence of the amount of cations (G) absorbed by peat on its dielectric constant was considered in [84]. It was found that the value of r'
moistened peat (N = 20%) with the initial additions of A1 and Na practically does not change, and with the absorption of Ca ions it decreases.
This decrease is apparently associated with a decrease in the mobility of sorbed molecules due to structural changes in the sorbent. The results obtained at relatively low frequencies (600 kHz) give reason to believe that the migration of ions in an electric field is not significant when the amount of cations absorbed by peat is within 0.2 mg/eq per 1 g of dry matter. Subsequently, with an increase in G, a “wave-like” change in r' is observed , which
An important issue in studying the dielectric properties of the sorbent - sorbed water system is, as noted above, the establishment of a connection between the experimentally determined macroscopic characteristics єs,
Г', r"
and the molecular parameters of the sorbent and sorbate.
The basis for establishing this connection can be the Onsager-Kirkwood-Fröhlich (OKF) theory, according to which the sorbent-sorbate mixture can be represented as a system of different sorbent and sorbate cells. For such a system, based on the general theorems of Froelich G6391,
the following relation was obtained [671]:
(es - I) (2es+ 1) 4 L
( Xch -
Chg,
- ^
-v -=¥ ■ <15-2>
S /=i /=i J
Where 8S is the static dielectric constant of the mixture; N ,-, Nf
- respectively, the concentration of sorbate molecules and sorbent cells
of j -
Go type;
R.; — dipole moment of the i-type sorbate molecule located in a certain configuration; p is the dipole moment of the sorbate cell of type I,
Located in a certain configuration; M is the average dipole moment of a sphere containing all types of sorbent and sorbate cells when the sorbed molecule of the i-th type is in a certain configuration;
Mr
is the average dipole moment of the sphere when the i-th sorbent cell is in a certain configuration.
Calculation of the value <ts, MU includes consideration of various configurations of the i-th molecule, i.e., various possible displacements of its charges, and determination for each configuration
Dipole moment M.
Calculation of
Мр
is performed for various configurations of the i-th sorbent cell.
Accurate determination of the value es of the sorbent-sorbate system using relation (15.2) is difficult, since the specific structure of the substance under study and the nature of the interaction between its particles are unknown. Therefore, relation (15.2) should be considered as the initial one for obtaining various approximate formulas with certain limits of application. In particular, if the change in the polarization of the sorbent during the sorption process is insignificant compared to the value of the polarization of the sorbate, then we can assume that
(esl— 1) (2esl+ 1) 4th +
51 /=і where 8si is the static dielectric constant of the dry sorbent.
Taking into account (15.2) we have:
(8S-1) (2b5+1) = (esl-l) (2ea+l) e+^V^fr, (,5.4)
8S BS1 R1^
Where Q =N '/N 0 ', No '
— concentration of sorbent cells in the absence of sorbate. For non-swelling materials 0=1.
Using the method described by G. Fröhlich [639], it is possible to isolate the contribution due to those displacements of electrons relative to nuclei whose natural frequencies lie in the optical range. In this case we have:
(Es - (2Es + "2) _ (Esl - N) (2Esl + YAM
8s 8s1
4l U
/=i
Where ha, ha are the refractive indices of the mixture and sorbent, respectively. 250
Included in formula (15.5), in contrast to (15.2), are dipole
The moments CG and M do not take into account the vibrations of electrons in the optical range. The natural frequencies of nuclear vibrations are in the infrared region. Since when the nuclei are displaced
The interaction of electrons with nuclei changes, the values p,/
And M, strictly speaking, also contain terms due to the electronic displacement. Taking into account the contribution corresponding to these displacements, we obtain:
(Є5 — Єоо) (2F—S -ь Єоо) ^ (6S1 — Єоо) (2Esl + Єоох)
Єя Єсі
4th V
KT
І= І
The values of p,/ and M in this relationship are determined by the orientation of the dipoles. In the case when the displacement of protons occurs under the influence of particles surrounding the CVS (molecules and
Dipole groups of the sorbent), dipole moments jut/ and M also contain terms describing proton polarization.
Formula (15.6) is more convenient for practical use in dielectric studies, since the values of Єоо and Єооі can be determined experimentally using the dielectric characteristics є' and є" [672].
To determine the value <р,/Л1> included in equation (15.4), it is necessary to perform the following operations: 1) for a fixed displacement of charges (except for electrons) of a water molecule that determine its dipole moment p,/, find the average value of the dipole moment of the entire environment; 2) taking into account the various possible shifts of charges of the sorbed mol-
Kuly, calculate the average value <р,/Л1>. Due to the complexity of such calculations, the approximate Kirkwood method is used in the theory of dielectrics. According to this method, only short-range interactions between the nearest neighboring molecules are taken into account, and the dipole moment M is defined as the vector sum of the dipole moment of the molecular
Kuly q and the average value of the sum of the moments of the nearest neighbors for a fixed p. For a liquid, taking into account the equivalence of all molecules and the directions of their dipole moments, Kirkwood’s theory allows us to obtain the following expression:
<|Ш>=к2(1+г<со8/>), (15.7)
Where z is the number of nearest neighbors of a given molecule, and is the average of the cosine of the angle between the directions of the dipoles of two neighboring molecules.
The disadvantage of the Kirkwood method, noted by Ya. I. Frenkel, is the fact that it is impossible to combine the idea of \u200b\u200bthe rotation of a molecule with its rigid connection with its neighbors. Therefore, when considering the orientation of dipoles in an electric field, it is necessary to take into account their retardation. Obviously, this inhibition significantly affects the polarization of sorbed molecules interacting with molecules of the solid sorbent, the mobility of which is much less than the mobility of the liquid molecules surrounding the dipole.
| (15.8) |
| Where GPC is the effective dipole moment of the i-th particle closest to the molecule (sorbate molecule or sorbent dipole group); fa - angle Between [x/ and t, r, r is the number of nearest particles that have formed an H-bond with a given water molecule. (The presence of a weaker van der Waals interaction of the molecule with other neighboring particles is taken into account, as noted above, by introducing the inhibition angle p.) |
| (15.9) |
In the absence of interaction between sorbed molecules, among the particles surrounding the molecule, one can distinguish the functional group of the sorbent with which it forms an H bond. This interaction determines the position of the molecule in the sorbent structure. The interaction of the molecule with other nearby sorbent particles, along with the H-bond, determines the orientational freedom or inhibition of the sorbed molecule. This inhibition can be taken into account based on the J1 model. N. Kurbatov [641], according to which the dipole moment of a sorbed molecule can be oriented within a certain solid angle. In the absence of an external electric field, all directions within this angle are equally probable. Obviously, such a model simplifies the actual interaction and movement of molecules, but it allows one to estimate the orientational polarization of molecules taking into account their inhibition. The contribution of such molecules to the polarization of the system is equal to the contribution of free, uninhibited molecules, the effective dipole moment of which is determined by the relation
Tsef=Tso[1 - cos4(P/2)],
Where [Ho is the dipole moment of the molecule located in the sorbent system -
Sorbate; p is the maximum angle of deviation [x0 from the axis of the cone formed by the solid angle.
Considering the sorbate molecules as free with a dipole moment q/ = ceph, in the Kirkwood approximation we obtain:
When calculating using formula (15.9), it is necessary to take into account the connection between the dipole moment ω of a molecule located in a vacuum and the dipole moment ω of a molecule located in a medium with an optical refractive index n [639]:
P* +
2
1*o =
——
H'O-
When calculating Јs for different sorption values, it is necessary to take into account the change in the polarization of previously sorbed molecules.
| (15.10) |
Relationship (15.9) was obtained for the orientational polarization of the sorbate. Taking into account proton polarization due to the transfer of the H-bridge proton is a more difficult task. Its solution goes beyond the scope of issues solved by the OCF theory. If, however, we assume that the transfer of the H-bridge proton occurs as a result of the orientation of surrounding particles, then the corresponding increase in the dipole moment of the molecule on the basis of classical statistics gives [673]:
| Where q — proton charge; |
| (15.11) |
Although it is currently not possible to estimate the values of AU
and I,
which depend on the interaction of SBC with surrounding particles, the obtained relations are useful in the analysis of dielectric sorption isotherms, since they relate the experimentally determined dielectric characteristics with the molecular parameters p,, fl, f, AU , I, d. The relatively large number of parameters makes it difficult to estimate them using relations (15.6) - (15.11). Therefore, when applying these relationships, it is necessary to make certain assumptions and select reasonable intervals of changes in the parameters under consideration. Such an attempt was made in Ref. [674] to estimate the proton polarization of water molecules monosorbed on a-PerO3.
An important issue in the study of the dielectric properties of the sorbent-sorbate system is the study of the frequency-temperature dependences of its dielectric characteristics. A detailed discussion of this issue is beyond the scope of this section. Let us only note that one of the urgent tasks of these studies is to decipher the dielectric spectra considered in the e' and z planes.
The resulting diagrams are described by various empirical relationships, discussed in detail in [672, 675].
It should be noted that when interpreting dielectric data and performing various calculations, additional information is needed about the sorbent - sorbed water system obtained using other physicochemical methods (NMR, IR spectroscopy, etc.). This can significantly increase the efficiency of studying the dielectric properties of moistened materials. At the same time, the high sensitivity of the dielectric method makes it possible to study the sorption of water on various materials in more detail. Further development of the dielectric method depends on establishing a closer and more specific connection with other physicochemical methods, as well as solving such pressing issues in the theory of dielectrics as deciphering dielectric spectra, calculating various types of polarization and dielectric characteristics of the sorbent - sorbed water system.
How to fix the situation
If you live in a private house, be sure to ground the wiring and, accordingly, if there is one, check the integrity of the circuit by measuring its resistance. Also make sure that the water heater or washing machine is working properly. First, disconnect them from the network and if the electric shocks stop, proceed to troubleshooting. Finding a breakdown in the wiring is quite difficult to determine without certain skills, however, if you wish, try to inspect all sockets, switches, and also check the wiring in the wall with a special tester. By the way, the sockets themselves in the bathroom must have a degree of protection of IP 44 and higher. It is better to place switches outside for reasons of electrical safety.
It is also necessary to protect electrical appliances with a residual current device, which will immediately turn off the electricity in the serviced area if it detects a current leak. For a bathroom, it is recommended to choose an RCD with a response characteristic of 10 mA.
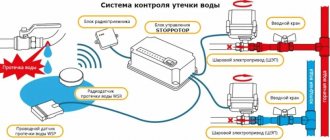
Additionally, we recommend installing a water leakage protection system in the bathroom. It will save your home and your neighbors’ homes from flooding during an emergency, or if you forget to turn off the water. In addition, the protective system can prevent electric shock through a puddle under the washing machine, for example, because the water will be shut off in a timely manner, and you will be informed about the accident. A popular water leak protection system is Neptun. You can read more about it in our review of Neptun Prow + Wi-Fi.
Another important point is the presence of a house-wide and individual potential equalization system, which combines all the pipes in the room, the sink and the bathtub itself with a grounding bus. Make sure that you have such a system in your apartment, and, just as important, check its functionality
Well, if you are convinced that the washing machine and boiler are intact, and the wiring is working properly, but at the same time there is an electric shock in the bathroom, feel free to go to your neighbors and find out what problems they may have in their apartment. If you are sure that they rewind the meter in this way or simply made an unsafe grounding, as a result of which you get an electric shock, contact your service organization, which will come with a check and find out what’s wrong. We talked about what to do if your neighbors steal electricity in a separate article.
That's all I wanted to tell you about this issue. We hope that now you know why the water from the tap, the walls, or the faucet itself is leaking in the bathroom and what to do to solve the problem!
Diagnostics
The first thing you need to do is turn off the electricity supply to the apartment at the switchboard. If your home is without power, but the problem has not disappeared, the neighbors are 100% to blame. Surely in every panel high-rise building there are craftsmen who unwind the electric meter. If there is no current in the tap, it means that the source of the problem is localized to you.
To find the cause, you need to turn off one by one all household electrical appliances that are somehow connected to water: washing machine/dishwasher, boiler, hob (if not grounded). Thus, by turning off these devices one by one, you can find the source of current supply to the tap. If everything is de-energized, but nothing has changed, the problem is either in the general grounding of the house or in your electrical wiring.
socket
Why does it give me an electric shock?
In fact, there are plenty of reasons, therefore, for better understanding, they should be divided into external and internal. The source of trouble in the first case is some kind of malfunction outside the apartment, in the second - within the housing. It happens that the electrical wiring is completely new, without problems, so in such a situation the following points are considered:
- Lack of common house grounding (as a rule, found in old buildings);
- Residents connected to a common riser use metal water pipes with a neutral wire. This example occurs if the electricity meter is “unwinded”;
- In the neighboring apartments the heating element of the water heater is faulty.
If we consider the above situations, the most common one is where neighbors want to save money by paying less for utility bills.
Of course, there will always be craftsmen who can not only “rewind” the electric meter, but also devices that take into account water consumption. But it should be understood that such tricks cannot be called safe for life.
Tension can occur in the following cases:
- Electrical wiring is faulty;
- Violation of wire insulation;
- Ungrounded sockets, water heaters, washing machines;
- The heating element of the boiler is faulty.
Water flowing from a tap produces electric current in another case - if an additional potential equalization system is provided using various non-ferrous metals. For this reason, professionals do not recommend direct contact between aluminum and copper - there will be a noticeable electrical impulse even with slight oxidation.
Other interesting questions and answers
What to do when you get an electric shock from tap water?
Guest2
If you do not want to suffer electric shocks from the faucet, you will have to do the following:
- Replace all sockets in the kitchen, bathroom and toilet with special models with a moisture protection class of at least IP44 or IP54. They will protect you from accidental breakdown of the socket body.
- Examine the wiring with an ohmmeter or a hidden break detector. Increased resistance in the area or a detector signal will indicate the location of the breakdown.
- Replace all problem areas of the wiring, or even better, replace the entire line leading into the bathtub, kitchen or toilet, using a special, moisture-resistant cable.
- Call a technician and ask to replace the heating element on the washing machine, dishwasher, and also on the boiler. Unless, of course, the source of the problem is water heating electrical appliances.
Vladimir1
The reason that a stream of water produces an electric current may be the lack of grounding on a washing machine, water heater or other electrical appliance that is connected to an outlet in the bathroom/kitchen or is located next to it. This may also mean that the wiring is faulty, a wire is twisted somewhere, or the wires are damaged. It happens that your neighbors have errors in the grounding of the water heating system, and an electric current is generated from the water from your tap. If you are sure that the problem is not in your apartment, then you need to look for neighbors who share a common riser to solve the problem. If in your bathroom or kitchen all the sockets and all the wiring are adapted to humidity, there are no problems with grounding and there is no damage to the electrical wiring and the appliances themselves, then contact the Housing Office/DUK to check the neighboring apartments for malfunctions. To determine your problem, call a specialist; it is better not to deal with electricity yourself, and this should be done as soon as possible. The electrician will conduct diagnostics and, if necessary, replace problem areas of wiring, switches and sockets, and indicate malfunctions of the water heating system and electrical appliances. It's good to have an electrician assigned to your home who may already be familiar with the problem if it's not a one-off problem. In addition, prices for services from housing office electricians are lower than from specialists from third-party companies. Natalya K.3
Why does tap water produce an electric current when the washing machine is turned on?
There are no intersections, they just stand next to each other. Leaks too. It hits little by little, barely noticeable on small wounds on the hands.
iris3
Either there is bad grounding here or there. In addition, there is an insulation failure somewhere. Let’s say that in a washing machine the insulation in some place is not good enough and a “bridge” has formed, through which electricity flows to the body. If the grounding is connected incorrectly or is missing, but there is a small voltage on the case. Water pipes, on the contrary, are well grounded and the resulting current goes into the ground. In this case, if you hold the washing machine with one hand and the water pipe with the other, then you are closing the electrical circuit. The wounds on the hand, as the most sensitive place, feel a small current. This can be dangerous because in a damp room the effect of the current is only enhanced due to worse insulation. You need to take a device (for example an ammeter) and check where the problem is coming from. Of course, it may turn out that everything is fine with your washing machine, but a small current flows from the side of the water supply system through some faulty electrical appliance.
Sveta17082
The water from the tap gives an electric shock... but only to me! Why?
Everything was fine before, but now... I’ll take a piss, wash normally, and two seconds before turning off the faucet shocks me! I turn off the tap and it goes crazy again! And the battery is hanging there. And after that, if I turn on the sink in the bathroom or kitchen for half an hour, the water flows out there. And only me! Grandma laughs and says that you need to sit on the phone less... But if it were the phone, it would ring immediately and constantly like... Alena .4
So some smart guy made a grounding connection to the water pipe. Seryozha3
Is water really a conductor of electricity?
Common sense tells us that we should not work with exposed electrical wires or even plug into an outlet while standing barefoot in a puddle of water or even just with wet hands. Therefore, most of us believe that water is a good conductor of electricity. Indeed, if we stand in it holding a live wire or a faulty electrical appliance in our hands, the water will increase the conductivity of your body and complete the electrical circuit, causing current to flow through you. This can lead to fatal injuries or even simple electric shock.
But in fact, pure distilled water does not conduct electricity at all. After all, there is nothing in it that could carry a charge. But, since it is an excellent solvent, it always contains some concentration of charged particles, no matter where in nature it is found.
Tap water always contains enough impurities, including minerals and chlorine, that it allows it to conduct electricity quite strongly. What makes water even more dangerous is that it can fill any open spaces between your body and any electrified wires or objects. The circuit will close even with a small amount of tap water on your body.
In addition, the salt from sweat will also dissolve in the water, increasing its conductivity. Impurities that can conduct electricity are called electrolytes. They include acids, salts and other substances. Electrolytes are divided into weak and strong according to the degree of their relative conductivity.
Security measures
Electrical wiring in wet rooms should include the installation of camouflage covers for sockets, switches, lighting fixtures and other elements of electrical appliances.
If water gets into the outlet, there is a possibility of an electrical leak. Then, when you touch the wall and the bathtub, an unpleasant tingling sensation occurs. Unfortunately, if these problems are not corrected, an injury from an electric shock is quite possible, with unpleasant incidents resulting in medical intervention.
To prevent current leakage, it is recommended to use only plug-in devices. Plug-in electrical appliances provide reliable protection against moisture. To ensure a 100% guarantee against moisture ingress, it is necessary to purchase high-quality electrical appliances, because often the stated characteristics do not coincide with the actual indicators.
When purchasing, carefully inspect the products and make sure they are tight and properly insulated. Despite the presence of housing protection, the devices must be installed in such a way as to minimize the ingress of splashing water.
When carrying out electrical wiring, they try to install the equipment so that the switches are located outside the bathroom. If possible, the distribution box should also be installed outside the room, where there is constant high humidity.
Sources of current leakage can be:
- the presence of a “warm floor” system;
- dysfunction of the vein structure of the heating cable;
- violation of the insulating elements of the heating device. In such cases, to eliminate the leak, it will be necessary to replace the entire “warm floor” system in the premises, since their structure is completely hidden under the floor screed.
If the passage of electric current was noticed in the premises, this does not mean that when troubleshooting the problem, the situation will not happen again
Therefore, you need to take care of precautions for the long term. The following safety measures are known today to ensure the safe use of electrical appliances:
- Installation of a residual current device (RCD) or a combined electrical device - a differential circuit breaker - into the apartment distribution panel. It is recommended to install such RCDs on devices that are most dangerous in case of electric shock.
- Installation of an incoming protective device in the distribution panel, which ensures safety in case of problems with the RCD. Such a device is considered a safety backup option.
- Presence of protective grounding in electrical wiring.
Sometimes it happens that when washing your hands, the water from the tap produces an electric shock due to neighbor's negligence. Recently, pipelines have often been used as a grounding conductor, although this is prohibited by law. If you suspect something like this, contact the supervisory organization immediately. Electricians must identify the cause and eliminate the consequences.
Troubleshooting Methods
The reasons why a broiler gets an electric shock can be very diverse:
1. Destruction of the heating element insulation. The source of this reason is the destruction of the shell of the heating element or periclase. When this shell is destroyed, the heating coil is exposed, which is constantly under high voltage. When the shell is destroyed, the spiral begins to actively interact with the best current conductor - water. As a result, slight electrical discharges are released, reminiscent of a slight tingling sensation. You can fix the breakdown yourself by replacing the worn heating element.
2. Incorrect connection of the boiler can also cause electrical discharges. Many people often confuse zero and ground, connecting the heating element incorrectly. To fix this problem, you need to reconnect the wires. Typically the ground cable is yellow-green.
After installation, it is important to check the wires not only outside, but also in the outlet. If in doubt, you should contact a specialist
Boiler connection
3. Touching a bare wire to the housing can also cause electrical discharges. This problem occurs if the insulation on any of the wires deteriorates or wears off. Electrical leakage occurs when a bare wire touches the computer case. To fix the problem, you need to disassemble the device and find the exposed wire that needs to be repaired or replaced with a new one.
Bare wire on TENE
4. The neighbor's water heater is faulty. This may also cause the problem. In this case, electricity will be transmitted through metal pipes. If such a situation occurs, turn off the boiler immediately. After turning it off, you need to touch the water: if it no longer stings, then the reason is in the neighbor’s boiler. Residents must be notified immediately of the breakdown.
What exactly happens in the water
Let's use your smartphone as an example of how water damage occurs and suppose you accidentally throw it into a puddle of water and this causes the device to become damaged and eventually crash completely. How exactly did the water cause all this damage?
Interestingly, it is not actually the water itself that is harming the device, but rather the microscopic impurities and ions in the water. These ions can bond together to form a conductive circuit that connects two points on the phone's chip. If the phone is turned on, it will send electricity where it should be, causing a short circuit and damaging the device.
Causes
Before you begin to fix the problem, you should understand the reasons for its occurrence.
- It is not uncommon in our conditions for neighbors to use a metal pipe as a neutral wire. Moreover, the current can break through periodically, so noticing such a problem can be quite difficult.
- Another common reason is that the insulation integrity of the outlet or wiring in the bathroom is broken. This problem is very common in rooms with old wiring.
- Current often flows if the washing machine is connected without grounding. She has access to water. This means that the current can “walk” not only in the bathroom, but also in the kitchen, for example.
- There is also a situation where an ungrounded electric water heater is connected. Then the current can strike through the water. For example, it is enough to open the mixer to let water flow and get a discharge. By the way, such a unit does not have to be in your bathtub; your neighbors can have it too. And you also have problems with current.
What is the electrical conductivity of water
The most common liquid on Earth has the ability to conduct direct or alternating current.
The electrical conductivity of water is a quantitative characteristic of this property, which is determined by the presence of charged particles - positive and negative ions. The latter include chemical elements that are part of the following organic and inorganic compounds:
- Alkalis.
- Salts of alkaline earth and other metals, primarily chlorides and sulfides (sulfates).
- Carbonates.
This indicator is higher, the more positively charged ions - cations - and negatively charged ions - in the liquid. Those. electrical conductivity is directly related to the salinity content of water. The specific electrical conductivity of water is inversely related to the resistance of water and is determined for the volume of liquid that is located in the gap between two electrons with an area of 1 cm2. The latter are located at a distance of 1 cm from each other.