The first alkaline batteries were put into production in the 60s, when manufacturers faced the issue of improving the quality and energy efficiency of batteries. They got their name from the electrolyte, consisting of an alkaline solution. The design of alkaline batteries involves the use of potassium or sodium hydroxide. Alkaline batteries are marked with the inscription Alkaline or Alkaline.
Alkaline batteries use potassium or sodium hydroxide in their construction.
Features of alkaline batteries
The electrochemical reaction in alkaline batteries is ensured by a mixture of zinc and manganese oxide. The same substances are included in saline batteries, but the alkaline solution significantly increases the service life of the device. Depending on the type of power source, the voltage is in the range of 1.5-12 V. At the same time, alkaline ones can operate at different currents.
Tablet-type alkaline elements are of minimal importance. They are distinguished by their miniature body and low technical performance. For example, batteries with a case shape of 164 have a capacity of only 8 mAh. More energy efficient cells like the PX625A have a capacity of up to 200 mAh.
Sizes and types of Duracell batteries
- Alkaline batteries:
- standard sizes AAA, C, D at 1.5v, as well as batteries 9V MN1604 (9v), 4.5v MN1203 (4.5v);
- similar-sized nickel-metal hydride batteries HR6, HR03, HR14, HR20 at 1.2v and battery HR9V at 8.4v;
- Power supplies for photographic equipment:
- lithium cylindrical 3 volts 123, 223 and CR2;
- lithium cylindrical 6 volt sizes 245 and 28L;
- lithium disk 3 volt 1/3N (diameter 11.6 mm and height 10.8 mm);
- lithium CR-V3 3 volt quadrangular shape 52x14x28 mm;
- alkaline disk 625A at 1.5 volts.
- Electronics batteries:
- lithium tablets 1220, 1616, 1620, 2022, 2025, 2032, 2430, 2450 at 3 volts;
- silver oxide disk element 357/303/R357/7 at 1.55 volts;
- alkaline tablets LR43, LR44, LR54 at 1.5 volts;
- alkaline cylindrical AAAA at 1.5 volts.
- For 1.55 volt silver oxide watches: 362/361, 364, 371/370, 377, 386/301, 389/381, 392/384, 394, 399/395.
- Alkaline cylindrical for safety devices:
- at 1.5 volts - N, J;
- for 12 volts - MN21, MN27.
The following power sources are most common in the CIS:
- cylindrical alkaline sizes: AA, AAA, C, D;
- 9 volt alkaline batteries (Krohn type);
- batteries and chargers;
- special ones marked MN21, MN27, 123, 28L, CR2;
- "Tablet" type.
Duracell tablets
The most common disk batteries intended for small medical devices, bank card readers, and small electronics are the following:
- lithium special sizes 2022, 2025, 2032 at 3 volts, which operate in the temperature range from -40 to +60 degrees and are often used in motherboards to maintain the functionality of CMOS memory;
- alkaline special LR44 at 1.5 volts, operate from -20 to +54 degrees.
All of them support Duralock technology, while lithium cells can be stored for up to 10 years, and the LR44 alkaline source can be stored for 5 years.
2032. They are marked DL2032 (according to the IEC standard - CR2032), have a capacity of 220 mAh, and weigh 2.8 grams. Dimensions: 20x3.2 mm.
2025. They are marked DL2025 (IEC – CR2025), capacity 150 mAh, weight 2.2 grams. Dimensions 20x2.5 mm.
2016. They are marked DL2016 (CR2016), capacity 75 mAh, weight 1.8 grams. Dimensions 20x1.6 mm.
Expert opinion
Viktor Pavlovich Strebizh, lighting and electrical expert
Any questions ask me, I will help!
However, you can make them yourself; craftsmen make such devices from cheap Chinese chargers for mobile phones. If there is something you don’t understand, write to me!
Design and principle of operation
The negative electrode of an alkaline battery is located in the center of the battery. It is a paste containing zinc powder, electrolyte and thickener. To protect internal elements from corrosion, the housing contains high-frequency zinc with the addition of third-party metals. This also allows manufacturers to exclude environmentally hazardous substances, such as mercury, from the composition.
The current conductor of the battery is provided by a brass rod passing through the powder. Thanks to it, the area of connection between the electrolyte and the electrode increases, which helps to reduce internal resistance and increase the discharge current.
The positive and negative electrodes are separated from each other by a porous structure, which is impregnated with the working solution. It also does not allow mixing pastes with different charges. The positive electrode consists of manganese oxide and graphite. It is this that fills the free internal space that remains between the membrane and the body.
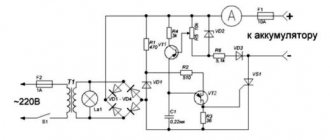
How to charge a battery: which ones you can, in a charger, at home.
- Through the power supply.
We connect it to the network and connect the battery with the connection wires, observing the polarity. It is important to prevent the battery from heating above 40-50 degrees. After this, turn off the power supply and let the battery cool. When it becomes warm, reconnect the power supply to the electricity for about 2 minutes, then place the battery in the freezer for 10 minutes. You can use it 2-3 minutes after removing it from the cold. A battery charged in this way can last for some time. - By heating method.
Is it possible to charge an alkaline battery?
Alkaline batteries are considered a highly efficient battery, so the question arises: can alkaline batteries be charged? This opportunity would allow you to save on the purchase of new batteries. However, according to manufacturers' recommendations, alkaline batteries should not be charged. This information is displayed on the packaging in the form of an inscription or pictogram.
You can find tips and instructions online on how to charge alkaline batteries in a charger. The operation is performed subject to time intervals, and the battery itself should not heat above 50 degrees Celsius. After the voltage increases, the battery also needs to be cooled. However, this method of recharging does not provide any guarantees and can lead to leakage of electrolyte, so you should not try it.
If it is not possible to replace used batteries with new ones, you can resort to the following methods to extend the performance of the power source:
Deformation of the battery case for recharging.
- Increase in case temperature. To do this, you need to dip the alkaline element in hot water for 30-40 seconds. The internal elements will receive some heat and release energy. It is strictly forbidden to heat a battery over a fire.
- Hull deformation. By squeezing the battery, you can achieve a slight increase in charge. In this case, it is necessary to monitor the degree of damage - the electrolyte should not leak out of the housing. Do not squeeze the battery with your teeth - this can lead to poisoning from the solution.
It is worth remembering that both methods are unsafe and allow you to achieve minimal effect. Therefore, it will be easier to buy new alkaline batteries.
What determines the speed of battery charging?
Significant factors affecting the battery charging speed are:
- ambient temperature, which should be in the range from -5°C to +50°C. The optimal option is 20°C-25°C;
- chemical composition of the energy source. Thus, nickel-zinc batteries require a special charger;
- amount of charge remaining.
In addition, the size of the charging current (the higher its value, the faster the battery will charge) and capacity (batteries with a small value of this parameter will be ready for use faster) have a noticeable impact.
For the process to be successful, you need to know how to properly charge batteries. The main rule is to thoroughly read the instructions and recommendations of the manufacturer of both the battery and the charger. The latter is strongly recommended to be chosen immediately when purchasing a battery.
What kind of lighting do you prefer?
Built-in Chandelier
HELP: During operation, the charger heats up, which is considered a normal consequence of the process. However, if the case is very hot, it should be immediately disconnected from the network.
Rechargeable batteries
In addition to alkaline, there are other types of batteries that can be recharged after use. Unlike standard batteries, reusable batteries allow you to use them for a long time with the help of a charger. Similar batteries are also available with an alkaline solution with a voltage of 1.5 V and a capacity of 150 mAh.
Battery charger.
The key advantage of an alkaline battery is that it is completely safe for humans and does not contain harmful substances. Such a battery holds a charge under storage conditions for many years, especially if it is not completely discharged. Instructions from manufacturers indicate that alkaline batteries can withstand up to 50 recharge cycles.
Reusable batteries have standard dimensions, standard sizes correspond to AA, AAA, C and D formats. They should be used in equipment with low current consumption, for example, in remote controls or flashlights.
Test results
Many people ask about which batteries are better, because it can be easy to get confused among numerous manufacturing companies, and not everyone can afford to constantly buy the same Duracell. Since AA and AAA batteries are so often used in children's toys, it is not surprising that both children and parents really want their furry mechanical friend to work much longer.
As already mentioned, among domestic analogues of alkaline elements in terms of capacity indicators, Cosmos is a good option. There are several companies in Russia that conduct a special test on batteries and, based on its indicators, help people choose the best of the inexpensive domestic options.
One of these. In order for the battery performance test to be truthful and accurate, six devices reminiscent of children's toys were taken as “test subjects”. They were placed in intensive operating conditions, with maximum energy consumption from the batteries.
The test showed that the discharge current was about 1000 milliamps. Various alkaline batteries were subjected to this discharge until the voltage level dropped to 0.9 volts. All indicators were recorded in a special table. The main “measure” of efficiency was the capacity of each element remaining after testing.
Among eight batteries from different manufacturers, the “Photon” and “Cosmos” brands participated in the experiment, the capacity of which, even after serious tests, remained at a decent level. Thus, if you want to purchase inexpensive alkaline elements that have good performance, you can ask for these brands in stores.
Testing has proven that these options are very convenient and cost-effective when lithium or more expensive alkaline batteries are not available.
Where did the idea to charge batteries come from?
This idea is not as absurd as it may seem. Yes, if the zinc cathode has dissolved, trying to revive it is stupid. But! Cheap batteries stop working due to oxides, and the chemical resource remains incompletely exhausted. So here it is. Soviet scientists also discovered that if an insulated battery is pierced with a strong current, the “crusts” fly off, the contacts are cleaned, and the reaction resumes.
Please note: The capacity of finger / little finger batteries is approximately the same, regardless of cost. Why do cheap ones sell out faster? Because their contacts and ionic solution are made of simpler materials.
Danger of charging batteries
The batteries contain caustic alkali. When charging, when electric current passes through it, the battery may explode. This must be taken into account when charging in a room, garage or other enclosed space.
What can happen when charging batteries.
Another nuisance that may arise during the charging process is electrolyte leakage. If a small amount of the substance comes into contact with the device, it will be damaged.
When purchasing galvanic cells, you need to take into account that only alkaline (alkaline) cells can be recharged. It is impossible to extend the service life of salt products. Recharging them is considered dangerous. They may explode, causing electrolyte to splash into your eyes.
How does the freezing method work?
- It is not recommended to recharge disposable batteries. This is ineffective and can damage the charger.
- Lithium power supplies (tablets) cannot be restored; they will explode if subjected to mechanical impact or attempts to open them.
- There is a way to restore alkaline batteries by refilling them with alkali. To do this, you need to disassemble the body, removing the brass rod, then pour in lye and put everything back together. This method works, but it is too complicated and not worth the effort. In addition, you can burn yourself with an alkaline solution.
Charge modes.
There are three types of charging batteries and batteries: normal, enhanced and forced.
Normal charge.
With a normal charge, batteries and rechargeable batteries are charged with a normal charging current for 6 hours. For lamella NK batteries, the normal charge current is numerically equal to one quarter of the nominal capacity. For some lamella-free batteries, the normal charge time may be more or less than 6 hours. For example, the normal charge time of the 2NKB-2 battery is 10 hours, and the 10NKB-60 battery is 4.5 hours.
A normal charge, which is carried out in order to monitor the capacity of batteries, is called a control charge.
Increased charge.
Enhanced charge is the charging of batteries and storage batteries with a normal or increased charging current for 12 hours. Enhanced charge is used to restore or maintain the nominal capacity of batteries and storage batteries in the following cases:
A). when brought into working condition;
b). in preparation for storage;
V). after changing the electrolyte;
G). after deep discharges below permissible final voltages (1 V per battery or battery cell) or discharges with low currents, alternating with long breaks in operation;
d). during irregular work.
The enhanced charge is carried out in one or two stages. In one stage, the batteries are charged with normal current for 12 hours. When charging in two stages, after 6 hours of charging with normal charging current, a break in charging is taken for 1-2 hours, after which the batteries are recharged with normal charging current for another 6 hours of normal charging charging with current takes a break in charging for 1-2 hours, after which the batteries are recharged with normal charging current for 6 hours.
Forced charge.
In case of emergency, to quickly bring the batteries into working condition, a forced charge mode is used, which provides for a reduction in the time of electrolyte impregnation and degassing of the batteries and batteries. Charging batteries and accumulators with currents whose magnitude exceeds the normal charge current.
Charging in forced mode reduces the service life of batteries and rechargeable batteries and therefore manufacturers allow, as a rule, no more than 10 charges in forced mode during the warranty period of their operation.
Only serviceable batteries are allowed to be charged in forced mode. After filling and impregnation with electrolyte, each element of such a battery (battery) must have an NRC greater than zero.
Procedure for performing work when charging alkaline batteries and rechargeable batteries.
Work on charging alkaline batteries and rechargeable batteries is carried out in the following sequence:
— prepare batteries for charging;
— calculate the electrical circuit for charging batteries;
— connect the batteries in series into charging groups;
—
prepare the chargers for operation in accordance with the operating documentation of the chargers and connect groups of batteries to the chargers;
— charge the batteries with a stabilized current, controlling the charge current, electrolyte temperature, electrolyte level, voltage on the battery cells and batteries;
— disconnect batteries from chargers after charging is completed;
— install batteries with open filling holes for degassing (removing gases from the internal volume of battery cells);
— bring the level and density of the electrolyte in the battery cells to normal;
— measure the voltage on each battery cell and fill out a maintenance log for batteries and batteries.
A very popular type of energy storage is alkaline batteries, due to their versatility. Caustic sodium or potassium is used as an electrolyte. Before buying such equipment, you will need to familiarize yourself with all the positive and negative sides, as well as the features of the device.
Charging methods
Before charging a AA battery at home, it is advisable to know what type of charger control you will need to use. Two charge control methods are used:
- by current;
- by voltage.
The first method is used for NiCd and NiMh batteries, and the second for lead-acid, LiIon and LiPol batteries. Automatic battery chargers using specialized microcontrollers allow you to properly recharge any type of energy cell and control the stages of energy recovery.
Charger with current control
Such devices are called galvanostatic. The main parameter of the memory is the battery current value. Correctly recharging the battery and not deteriorating its characteristics can be achieved by selecting the current value and charging speed. In order to determine the current values, the equality I = 0.1C is used, where C is the battery capacity. It is not difficult to understand why it is not recommended to use a larger value by imagining the chemical processes taking place in galvanic devices. In addition, firstly, there is increased heating, and secondly, there is a memory effect.
To avoid self-discharge, chargers usually switch to low-current charging mode at the end of the charge.
But this method is unacceptable for alkaline batteries, so they cannot be recharged in this mode. For these types, a charging termination method is used when the current does not change for several hours.
Voltage control method
The type of operation is based on a potentiostatic mode that turns off the charging process when a certain voltage is reached. For this type of charger, different charging rates are used. For nickel-cadmium and nickel-metal hydride, three charge rates are used: long (0.1C), fast (0.3C) and ultra-fast (1C). During the charging process, the current decreases, and the voltage at the battery terminals approaches the charger voltage. It is believed that this method cannot fully charge the battery.
Advantages and disadvantages
The strengths of alkaline battery include:
- For operation, a much smaller amount of electrolyte is required than in the case of salt batteries;
- Successful design: the chemical reaction occurs over a sufficiently large area;
- There is practically no release of gaseous substances;
- Quite a long shelf life and low risk of losing the initial charge;
- Ability to work at low temperatures;
- Resistance to intense work with increased current consumption;
- Discharge occurs relatively evenly during use.
However, it would be a mistake to classify this product as ideal, because it is different:
- Quite a high price compared to other types of chemical power sources;
- Relatively high weight;
- The vast majority of commercially available products cannot be recharged without the risk of an explosion of the gas inside.
Storage rules
Devices are available for long-term or temporary storage. When purchasing a new battery, be sure to check the plugs for their fit.
We recommend: Repair and restoration of Ni-Cd batteries
Great attention must be paid to checking the valve rubber. The operation of the entire battery will depend on its condition.
To prevent the paintwork from peeling off, the body must be treated with lubricant.
For proper storage, you will need to perform a number of actions:
- Discharge to 1 volt.
- Pour out all the electrolyte.
- Remove dust, dirt and salt.
- Renew the varnish coating.
If the battery will remain idle for 1-10 months, it is necessary to periodically check its performance. All plugs must be pressed tightly against the device. Transportation can only be carried out if the battery is prepared for long-term storage. It is not advisable to store alkaline batteries with other power sources.