For a long time, the acid battery was the only device capable of providing electric current to autonomous objects and mechanisms. Despite the high maximum current and minimal internal resistance, such batteries had a number of disadvantages that limited their use in devices that consume large amounts of electricity or in enclosed spaces. In this regard, lithium-ion batteries lack many of the negative qualities of their predecessors, although they do have disadvantages.
What is a lithium ion battery
The first lithium batteries appeared 50 years ago. Such products were a regular battery, in which a lithium anode was installed to increase the level of electricity output. Such products had very high performance characteristics, but one of the most serious drawbacks was the high probability of lithium ignition when the cathode overheated. Given this feature, scientists eventually replaced the pure element with metal ions, as a result of which safety increased significantly.
Modern li-ion batteries are very reliable and can withstand a large number of charge and discharge cycles. They have minimal memory effect and relatively light weight. Due to these properties, lithium batteries are widely used in many devices. The product can be used as a battery, in the form of batteries for household appliances, and also as a highly efficient traction source of electricity.
Today, such devices have several disadvantages:
- high cost;
- do not like deep discharges;
- may die at low temperatures;
- lose capacity when overheated.
As more advanced analogues, you can find lithium polymer or lithium titanate batteries, but they are noticeably more expensive.
Protected and unprotected lithium-ion batteries
It is also important for lithium batteries whether they are protected or not. The operating range of the former is 4.2-2.5V (used in devices designed to work with lithium-ion sources): LED flashlights, low-power household appliances, etc.
Power tools, bicycles with electric motors, laptops, video and photographic equipment use unprotected batteries controlled by a controller.
We recommend:
- Selecting a battery for an uninterruptible power supply
- Universal battery charger
- Wismec rx200 mod: review and specifications
How is li-ion battery production carried out?
Lithium-ion batteries are produced in several stages:
- Manufacturing of electrodes.
- Combining electrodes into a battery.
- Installing the protection board.
- Installing the battery into the case.
- Filling with electrolyte.
- Testing and charging.
At all stages of production, technology and safety measures must be observed, which ultimately allows us to obtain a high-quality product.
Lithium-ion batteries use foil as a cathode with a lithium-containing substance deposited on its surface.
Depending on the purpose of the battery, the following lithium compounds can be used:
- LiCoO2;
- LiFePO4;
- LiNiO2;
- LiMn2O4.
When producing cylindrical power sources of size AA and AAA, the main electrode is rolled into a roll, which is separated from the anode by a separator. With a large cathode area, the film of which has a minimum thickness, it is possible to achieve high energy intensity of the product.
Converting capacity from Ah to Wh
To calculate the absolute constant energy capacity in Wh, knowing the value in Ah, you need to multiply it by the rated voltage of the battery:
Wh (Wh) = V (V) x Ah (Ah).
For example, for a battery with a capacity of 13 Ah and a voltage of 36 V, the absolute energy capacity will be 13 Ah x 36 V = 468 Wh.
Similarly, knowing the absolute constant energy capacity of a battery in Wh, you can calculate its actual capacity at a certain operating voltage of the equipment. To do this, simply divide the absolute capacity in watt-hours by the operating voltage of the load in volts:
Ah (Ah) = Wh (Wh) : V (V).
For example: 468 Wh :36 V =13 Ah, and 468 Wh :24 V =19.5 Ah.
Operating principle and design of a li-ion battery
A lithium ion battery works as follows:
- When direct electric current is applied to the battery contacts, lithium cations move into the anode material.
- During the discharge process, lithium ions leave the anode and penetrate into the dielectric to a depth of 50 nm.
In the “life” of a lithium-ion battery, there can be up to 3,000 such cycles, while the battery can deliver almost all the electric current accumulated during the charging process. A deep discharge does not lead to oxidation of the plates, which makes such products stand out compared to acid batteries.
Not all li-ion batteries tolerate deep discharges well. If such a battery is installed in a phone or camera (AAA type), then if it is deeply discharged, the controller board blocks the ability to charge the battery for safety reasons, so it will not be possible to charge it without a special charger. If this is a traction lithium battery for a boat motor, then it will not be at all afraid of a deep discharge.
Unlike AA batteries, complex batteries consist of several separate sources of electricity connected in parallel or in series. The connection method depends on what electricity indicator needs to be increased.
It's just a bomb-2. Li-Ion - how not to take off
Over the past ten years, lithium-ion batteries have moved from expensive exotics to the category of the most common autonomous power sources. It is not surprising that they have become popular in the hands of DIYers, including beginners. Sometimes the technical solutions in their creations make your hair stand on end - after all, the peculiarity of batteries of this type is their increased danger, primarily fire hazard. My story is about how to properly “cook” this “fugu fish” so that no one burns or explodes.
Previous article on the “explosive” topic is here.
The operating principle of a lithium-ion battery.
Chemical current sources based on lithium have been widespread for a long time. Already at the end of the 20th century, lithium batteries were firmly established in watches, calculators, computer motherboards, and remote controls. According to the principle of operation, they are not much different from manganese-zinc cells, with the exception that lithium replaces zinc, and instead of an aqueous solution of alkali or ammonium chloride, an electrolyte based on non-aqueous solvents, such as propylene carbonate or thionyl chloride, in which the lithium salt is dissolved , dissociating to form a lithium ion, which carries current in such an electrolyte. But replacing zinc with lithium led to the voltage increasing from one and a half to three volts, and the energy intensity increased several times. At the same time, the chemically inert organic electrolyte and the high degree of tightness of the structure reduced self-discharge to almost nothing - delivering microampere currents, such a battery can operate for decades.
Do you know why you can't charge regular batteries? It would seem that when current flows in the charging direction, processes will occur on the electrodes “in the reverse order”: zinc will be deposited on the negative electrode, and on the positive electrode the active mass, which was once manganese dioxide and has given up its oxygen, will be oxidized again, again turning into fresh MnO2. But everything is spoiled by the fact that simultaneously with these processes, the water in the electrolyte also decomposes. The released gases inflate the battery case and squeeze the electrolyte out with dire consequences for the equipment.
There is no water in a lithium cell. Propylene carbonate, which serves as a solvent, is not subject to electrolysis, so such a cell can be charged without adverse reactions. However, such a lithium battery did not take off. Or rather, he was just taking off - into the air. Lithium did not want to lie on its anode in a neat thin layer, but crystallized in the form of needle-shaped crystals - dendrites. Exactly the same dendrites, by the way, are formed when trying to charge a manganese-zinc battery, but it was in a lithium battery that they led to disaster. Sooner or later, such a dendrite would bridge the gap between the anode and cathode and cause a short circuit. The flowing current heated both the cathode mass, from which oxygen was released, and the lithium, which ignited in this oxygen, and the separator, which simply ceased to exist, after which the lithium, electrolyte and cathode mass - fuel and oxidizer - turned into a hellish mixture. As one friend of mine, an inventor involved in these experiments, told me, the military, for whom they were trying to create these batteries, lost all interest in them as sources of current, but the regular powerful explosions, accompanied by a blinding red (from lithium) flame, fascinated them and Each time the military wondered if this explosive could be used somewhere.
They also worked in this direction abroad, and even achieved something by using mechanically stronger ceramic separators, special charging methods, and special additives to the electrolyte. But still, the danger of dendrite formation remained - such a battery was too dangerous for its practical use if it exceeded the size and capacity of a tiny watch battery.
The breakthrough came from two discoveries. The first is the discovery of the ability of some complex oxides and sulfides containing lithium to donate and absorb back lithium ions at the cathode. The second is the ability of compounds of a layered structure (graphite, molybdenum disulfide) to reversibly absorb significant amounts of lithium into the interlayer space (up to a compound of the LiC6 composition), capturing its atoms immediately after the discharge of Li+ ions at the anode and preventing its release in metallic form, and therefore preventing formation of dendrites. These discoveries and the invention of the lithium-ion battery were awarded the Nobel Prize last year. Its laureates are M.S. Whittingham, the discoverer of the phenomenon of lithium intercalation into titanium and molybdenum disulfides, who first proposed the use of this phenomenon in batteries, J. Goodenough, who studied the reversibility of the absorption and release of lithium ions by lithium cobaltite at the cathode, and, in fact, the inventor of the lithium-ion battery, Akira Yoshino.
The operating principle of Akira Yoshino's lithium-ion battery, which he invented in 1991, is as follows. Singly charged lithium cations are practically the only ion that carries current in an organic non-aqueous electrolyte. A counterion is a bulky and inactive molecular “construction” that has a negative charge.
When charging the battery, the Li+ ion is discharged on the surface of the graphite anode, turning into a neutral lithium atom. This atom is immediately absorbed by graphite, penetrating between the layers of its crystal lattice. Lithium graphitide is formed - the so-called intercalate or interstitial compound. In terms of its chemical properties, it is a strong and active reducing agent.
At the same time, lithium cobaltite at the cathode supplies lithium ions into the solution, and at the same time, losing lithium, its composition is increasingly approaching cobalt dioxide, as a result of which it becomes a strong and active oxidizing agent.
The electrochemical potential difference between these oxidizing and reducing agents is equal to the emf of a lithium-ion battery.
During discharge, reverse processes occur. Lithium, leaving the interlayer space on the anode, gives up an electron to the external circuit and acquires a charge, becoming a cation, and lithium graphitide - simply graphite. At the cathode, these cations return to the vacancies of the lithium cobaltite crystal lattice, which loses its oxidizing properties by accepting an electron into the external circuit.
Due to the absence of side processes, this electrochemical system has a very high degree of reversibility and for this reason is characterized by excellent efficiency.
Lithium polymer batteries are not, as many people think, a separate type of battery. Instead of a liquid electrolyte, they use a polymer-based gel electrolyte, and all electrochemical processes in them are no different. The absence (or rather, a minimal amount) of liquid electrolyte makes it possible to give them almost any shape and, instead of a durable metal case, place them in polymer film cases in the form of a sealed bag, which, among other things, increases the energy storage density.
There are also varieties of lithium-ion batteries with different electrochemical systems, such as lithium iron phosphate and lithium titanate. Their operating principle is the same, but the materials of the cathode mass are different and, accordingly, the voltages are different. The specific capacity of these batteries is lower than that of the classic cobalt lithium-ion system, but they surpass them in service life, ability to deliver current at low temperatures and, according to manufacturers, safety.
Actually, safety is perhaps the main “trouble” of lithium-ion batteries.
Hidden threat
Alas, having “tamed” lithium, Akira Yoshino did not turn this fiery lion into a harmless little mouse. And how can one expect complete safety from a device in which, I repeat, a strong and active oxidizing agent is adjacent to an equally strong and active reducing agent and they are separated only by a few tens of microns of a porous polymer separator film? Once this film becomes weak somewhere, allowing a short circuit, the avalanche-like process of self-heating and self-destruction cannot be stopped. The contents of the battery turn into an explosive mixture of fuel and oxidizer. And this mixture has already been set on fire.
The fact that lithium-ion batteries generally do not explode is due to the many precautions that are taken when using them. They are not observed by the user - this is monitored by automatic electronic devices. Where a lithium-ion battery is used, there is no place for the simplest chargers from the world of “lead” and “nickel-cadmium”. The charger must be “smart”. The process of charging a lithium-ion battery is multi-stage, requires strict adherence to parameters and must be completed on time, and it is absolutely unacceptable to shift responsibility for this to the user, since his forgetfulness in this case can lead to a fire or explosion.
The fact is that the absence of side processes in a lithium-ion battery is not absolute. In order to avoid them, you need to stay within a certain “safe” territory. So, at a voltage above 4.2..4.5 V or at too high a charge current, graphite no longer has time to “absorb” lithium, and it forms a metallic phase. The same thing happens if graphite loses its active surface, which happens, for example, due to overdischarge. As soon as metal appears on the surface, it begins to form dendrites and... you can call the fire department. Finally, overvoltage can cause electrolysis of the electrolyte components (including uncontrolled impurities) and the release of gases, the pressure of which can break the seal of the battery, which also poses a risk of fire - the compound of lithium embedded in graphite spontaneously ignites in air.
Overload during discharge is also dangerous. Overheating by the discharge current can cause boiling or thermal decomposition of the electrolyte, release of oxygen from the cathode active mass, and damage to the separator. The result is the same: short circuit and fire. Mechanical damage to the battery will have the same effect.
It is good practice not to rely on the reliability of the charger. In the vast majority of industrially produced devices (with the exception of “marginal” cases such as electronic cigarettes and model airplanes) containing lithium-ion batteries, regardless of the controller that is assigned charge functions, there is another controller that performs protection functions. In its simplest version (for example, on the DW01A chip, which is the basis of the protection boards for almost all Chinese batteries), it turns off the battery when it is overcharged (exceeding the permissible voltage), overdischarged, too much charging and discharging current, or overheating. In more complex cases, these basic functions are supplemented by balancing the battery (if it consists of several cells connected in series), monitoring its “health,” counting ampere-hours during charging and discharging (which allows you to determine the remaining percentage of charge much more accurately than with simple voltage measurement) and other functions. This controller - it is called the Battery management system (BMS) or simply “protection board”, as a rule, is an inseparable part of the battery, being in the same case with it and being tightly soldered to its terminals.
There is also a third level of protection. This is a mechanical device that breaks the circuit when the pressure or temperature inside the battery “can” increases. Unfortunately, it is not a panacea, since in many cases heating and gas evolution begin after the battery fire can no longer be stopped.
By the way, a typical figure for LiIon is 250 Wh/kg or 0.9 MJ/kg. This is only four times less than the energy reserve in explosives such as TNT. In a powerful laptop, the TNT equivalent of a battery can be compared to a hand grenade. So lithium-ion batteries are no joke. Their explosion could well lead to the death and injury of many people.
You can find many videos and photographs of explosions and fires of lithium-ion batteries on the Internet. I hope they convince you that everything is more than serious.
Charge and discharge correctly
Now let's figure out how to properly charge these dangerous lithium-ion batteries so that they are not so dangerous.
The generally accepted one, recommended by all lithium-ion battery manufacturers, is the CC-CV algorithm. This means that charging begins with a stabilized current, and when a certain voltage is reached, it then stabilizes at this level. This method is close to the method of charging lead batteries, differing from it only in the mode.
For most standard lithium-ion batteries, the transition voltage from the CC stage to the CV stage at room temperature is 4.20 V. Some older batteries with a coal coke anode should only be charged to 4.10 V, while these are becoming more common in recent years. “high-voltage” batteries that allow charging up to 4.35 and even 4.45 V. A slight excess of this voltage causes a sharp reduction in service life, and a more significant excess leads to fires and explosions. The required accuracy of setting the threshold voltage for standard batteries is ±50 mV, and for “high-voltage” batteries, the higher the voltage, up to ±5 mV with a threshold voltage of 4.45 V. Of course, a lower voltage only leads to a decrease in the available capacity, and An increase in voltage is unacceptable under any circumstances.
The standard charging current is considered to be 0.5C, and most batteries can be charged with a current of up to 1C without damage, and some allow higher currents provided that overheating is avoided. C here is the current in amperes, numerically equal to the capacity in ampere-hours. But this current cannot be used to charge deeply discharged batteries, the voltage at the terminals of which has dropped below 2.9-3.0 V. In this case, a precharge stage is necessary - the battery is charged with a current of 0.05-0.1 C until the voltage reaches three volts. But batteries that are too deeply discharged cannot be charged at all. The charger should not allow the battery to be charged if the voltage at its terminals has dropped below 2.5 V. With such a deep discharge, the battery usually loses a lot of capacity, but this is not so bad: its charge is associated with the danger of lithium metallization and fire. By the way, “high-voltage” batteries are more sensitive to deep discharge, and they should not be allowed to discharge below 2.75 V.
During the CV stage, the current decreases exponentially. At this stage the battery should not remain indefinitely. The charge should be automatically stopped after the current drops to 0.05-0.1C.
It is preferable to implement such a multi-stage charging algorithm on specialized controller chips. There are currently many such controllers produced, both independent (typical examples are the well-known LTC4054-4.2, TP4056, TP5000, etc.) and built into multifunctional power controllers, including several switchable linear and pulse voltage converters, like The RK819 chip used in many mobile devices.
A bad, very bad practice is to use conventional integrated linear and switching stabilizers for this purpose, and in particular the popular modules sold as “boards for charging Li-Ion” from Aliexpress on LM2596, XL4015, etc. This is exactly what they often do when converting screwdrivers to lithium batteries, not taking into account the danger that over time the voltage set at the output may “go away” due to the low quality of trimming resistors on these Chinese boards. If the motor of this resistor loses contact with the resistive element, the output will simply be the input voltage. And this is not to mention the fact that without external circuit solutions such a “controller” will not turn off the battery when charging is complete and will not provide precharge of a heavily discharged battery with a low current. In any case, when designing and assembling a charger for lithium-ion batteries, you should think about reliability. A malfunction here can be very costly, sometimes in human life.
Another extremely unfortunate solution, found in the practice of DIYers and even the “Chinese”, is to charge a battery equipped with a protection board before it is triggered. Firstly, the BMS turns off the battery when the voltage is exceeded. Secondly, with such charging, without the CV stage, only part of the capacity is used. Paradox: the battery is simultaneously over- and under-charged.
As a last resort, you can charge lithium-ion batteries with a current of 0.1 C until they reach 4.10..4.15 V, followed by a cutoff. But, according to some data, presumably, this mode has a bad effect on the current output and service life of the batteries.
Lithium-ion batteries tolerate not only overcharging, but also overdischarge very poorly. A voltage of 2.5 V per cell and below is fatal - such a battery is already dangerous to charge. And the region between 2.5 and 3 V, which, although formally acceptable, should be avoided if possible, since this negatively affects the service life. In a device powered by lithium-ion batteries, a forced shutdown should be provided when the voltage drops to 3 V. By the way, the vast majority of smartphones turn off already at a voltage of 3.35..3.4 V, since their power controllers use only step-down converters voltage, and at a lower voltage it is impossible to generate a voltage of 3.3 V. Therefore, all the advice “put the phone on charge without waiting for it to turn off, as this is very harmful to the battery” is not true. Such a high cut-off voltage, of course, slightly reduces the usable capacity, and at the same time slightly extends the battery life.
Balancing
The charging process becomes more complicated if we are dealing with a battery of series-connected elements. The fact is that no two batteries are alike. If the capacity of one of them is slightly larger and the other is slightly smaller, the voltage on the latter will increase faster than on the former. In this case, if we charge the battery to 8.40 V, this battery will end up a little overcharged. Over time, these small overcharges will lead to faster wear, which means that the voltage on this battery will increase more and more each time. A “snowball” of increasing battery imbalance occurs, which can result in an explosion.
To prevent this, it is necessary to control the voltage not only of the entire battery, but also of each element separately, preventing the voltage of each of them from exceeding. Typically, one or another balancing circuit is used that shunts the “advanced” elements during charging, when they reach the maximum voltage. These are so-called passive balancing schemes. Obviously, during their operation, part of the energy is dissipated in the form of heat, which significantly reduces the charging efficiency and worsens the thermal conditions inside the battery assembly. More efficient and better use of capacity are active balancing methods, which ensure energy transfer from the terminals of an already charged “can” to those that are still undercharged.
The figure shows the simplest scheme for balancing a battery of two elements on two comparators (https://power-e.ru/hit/sistemy-balansa/). Typically, such systems are implemented on specialized microcircuits, such as the LTC3300-1, and are included in the BMS, remaining connected to the battery at all times. Such controllers have a wide range of functions, including not only balancing, but also monitoring the condition of the battery during its service life.
Active balancing circuit on LTC3300-1 (Rykovanov A. Balance systems for Li-ion batteries // Power Electronics. 2009. No. 1
Currently, intelligent balancing systems have become widespread, making better use of battery capacity due to a compromise distribution of charging current, which is determined by the actual capacities of each of the elements, measured in previous cycles.
How to handle, store, where to put leftovers
Based on the above, lithium-ion batteries should be handled with care. Risk of fire and explosion occurs due to improper charging, short circuit and mechanical damage. The latter is especially true for lithium-polymer batteries that lack a durable protective case. By accidentally or intentionally puncturing or tearing the film protecting the battery, you can end up with a blinding red fire in your hands within 10-15 seconds. The same can happen when the battery is bent and squeezed, and especially if it is pierced through with any tool. This happens when you try to remove a battery glued with double-sided tape from a mobile phone to replace it with a new one. The risk is reduced by removing the dead battery, so this should be done before starting work. For the same reason, and also due to the fact that when shorted it can produce tens, if not hundreds of amperes of current, such batteries should be stored securely and neatly packed, and not in a heap of radio junk.
In general, these batteries should be brought to a charge level of 30-50% before storage. They should be stored at room temperature. Otherwise, some “experts” claim that they need to be kept in the refrigerator. No need. But under no circumstances should you store old, dead, and especially swollen batteries; you need to get rid of them as soon as possible, since they are unpredictable and can cause a fire at any time.
The question of “where to dispose of it” is quite complicated. Considering the environmental hazard of lithium (according to the maximum permissible concentration is close to lead), they should be disposed of by special organizations, but in our country I do not know such organizations that work with private individuals. They should not be thrown into the trash, and especially not into battery containers. Perhaps the ideal option is some kind of lockable box with sand in the open air, the contents of which would be collected by special services...
You can’t (and if you really want to, then you can’t either!) try to solder batteries. Spot welding only! The exception is lithium-polymer batteries with specially elongated solder pins and cylindrical batteries with pre-welded ribbon lamellas. Even slight overheating can lead to depressurization with subsequent self-ignition, and to melting of the separator and internal short circuit.
All sorts of shamanism like “push the battery” or “unlock the controller” is a risk that there will be a fireball in your hands, in your pocket or in your bed. Remember, if the battery controller is stuck, it's not because the money-hungry manufacturer wants you to buy a new one. This is because the manufacturer is reluctant to pay for the damage caused by batteries that catch fire.
Having assembled the charger (it doesn’t matter - as an independent product or as part of any structure), you need to carry out the first charge cycle, connecting a voltmeter and a milliammeter along with the battery, and making sure that it works correctly. Moreover, pay attention to the accuracy of the measurements: the maximum permissible voltage deviation from the nominal 4.2 V does not exceed 1.2%, and the error of common inexpensive multimeters with a capacity of 3.5 digits when measuring this voltage at a limit of 20 V reaches 1%.
When assembling a battery from several batteries, you need to select elements that are as close as possible (within 1-3%) in terms of capacity when connected in series, and in terms of internal resistance when connected in parallel. Before connecting elements in parallel, you need to equalize their voltage. Battery cells must be strictly from the same batch.
You cannot repair a battery by replacing one element with a new one. In this case, imbalance is practically guaranteed. And you already know what the imbalance threatens (hint: fire and explosion).
A fuse is something that should be present in any lithium-ion battery circuit.
And once again - be careful and careful.
Sizes and types of li-ion batteries
Lithium-ion batteries have become widespread. Such sources of electric current are used in various household devices, gadgets and even cars. In addition, industrial lithium-ion batteries with large capacity and high voltage are manufactured. The most popular types of lithium batteries are:
| Name | Diameter, mm | Length, mm | Capacity, mAh |
| 10180 | 10 | 18 | 90 |
| 10280 | 10 | 28 | 180 |
| 10440 (AAA) | 10 | 44 | 250 |
| 14250 (AA/2) | 14 | 25 | 250 |
| 14500 | 14 | 50 | 700 |
| 15270 (CR2) | 15 | 27 | 750-850 |
| 16340 (CR123A) | 17 | 34.5 | 750-1500 |
| 17500 (A) | 17 | 50 | 1100 |
| 17670 | 17 | 67 | 1800 |
| 18500 | 18 | 50 | 1400 |
| 18650 (168A) | 18 | 65 | 2200-3400 |
| 22650 | 22 | 65 | 2500-4000 |
| 25500 (type C) | 25 | 50 | 2500-5000 |
| 26650 | 26 | 50 | 2300-5000 |
| 32600 (type D) | 34 | 61 | 3000-6000 |
The first two digits of such designations indicate the diameter of the product, the second pair - the length. The last “0” is placed if the batteries are cylindrical in shape.
In addition to cylindrical batteries, the industry produces Krona batteries with a voltage of 9v and powerful industrial batteries with a voltage of 12v, 24v, 36v and 48v.
Battery for stacker
Depending on the elements that are added to the product, the battery case may have the following markings:
- ICR – containing cobalt;
- IMR — — — — manganese;
- INR — — — — nickel and manganese;
- NCR - - - - nickel and cobalt.
Lithium batteries differ not only in size and chemical additives, but primarily in capacity and voltage. These two parameters determine the possibility of their use in certain types of electrical devices.
Where are li-ion batteries used?
Lithium-ion batteries have no alternative where a battery is needed that can deliver almost all of the electricity and perform a large number of charge/discharge cycles without reducing capacity. The advantage of such devices is their relatively low weight, because there is no need to use lead gratings in such devices.
Given their high performance characteristics, such products can be used:
- As starter batteries. Lithium batteries for cars are becoming cheaper every year, thanks to new developments that reduce production costs. Unfortunately, the price of such batteries can be very high, so many car owners cannot afford such a battery. The disadvantages of lithium-ion batteries include a significant drop in power at temperatures below minus 20 degrees, so in northern regions the operation of such products will be impractical.
- As traction devices. Due to the fact that lithium-ion batteries can easily withstand deep discharge, they are often used as traction batteries for electric boat motors. If the engine power is not too high, then one charge is enough for 5 - 6 hours of continuous operation, which is quite enough for fishing or taking a boat trip. Traction lithium-ion batteries are also installed on various loading equipment (electric stackers, electric forklifts) operating in enclosed spaces.
- In household appliances. Lithium-ion batteries are used in various household devices instead of standard batteries. Such products have a voltage of 3.6v - 3.7v, but there are models that can replace a regular salt or alkaline battery with 1.5 Volts. You can also find 3v batteries (15270, CR2), which can be installed instead of 2 standard batteries.
Such products are used mainly in powerful devices in which conventional salt batteries discharge very quickly.
Traction battery
Rules for using li ion batteries
The service life of a lithium battery is influenced by many factors, knowledge of which will significantly increase the resource. When using this type of battery you must:
- Try not to let the battery drain completely. Despite the high resistance of the battery to such influences, it is advisable not to squeeze all the “juices” out of it. Particular care should be taken when operating these batteries with UPS and high power electric motors. If the battery is completely discharged, it is necessary to immediately revive it, that is, connect it to a special charger. You can boost the battery even after a long stay in a state of deep discharge, for which you need to perform a high-quality charge for 12 hours, then discharge the battery.
- Avoid overcharging. Overcharging negatively affects the performance of the product. The built-in controller is not always able to turn off the battery in time, especially when charging is carried out in a cold room.
In addition to overcharging and excessive discharge, the battery should be protected from excessive mechanical stress, which can cause depressurization of the case and fire of the internal components of the battery. For this reason, it is prohibited to send by mail batteries containing more than 1 g of pure lithium.
Used as a battery for screwdrivers, laptops and phones
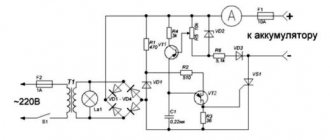
Diagram of a device for charging batteries
It looks like this:
The circuit is distinguished by its reliability and repeatability, and the included parts are inexpensive and easily accessible. To increase the battery life, proper charging of li-ion batteries is required: towards the end of charging, the voltage should decrease.
After its completion, i.e. When the current reaches zero, charging of the li-ion battery should stop. The circuit given above satisfies these requirements: a discharged battery connected to the charger (VD3 lights up) uses a current of 300 mA.
The ongoing process is indicated by the burning LED VD1. The current gradually decreasing to 30 mA indicates that the battery is charging. The end of the process is signaled by the lit LED VD2.
The circuit uses an operational amplifier LM358N (you can replace it with an analog KR1040UD1 or KR574UD2, which differs in the location of the pins), as well as a transistor VT1 S8550 9 LEDs of yellow, red and green colors (1.5V).
How to store lithium ion batteries
If there is a need for long-term storage of lithium-ion batteries, then to minimize the negative impact on the products, you must adhere to the following recommendations:
- Store the product only in a dry, cool place.
- The battery must be removed from the electrical device.
- The battery must be charged before storage. The minimum voltage at which internal corrosion processes will not form is 2.5 Volts per 1 element.
Considering the low self-discharge of such batteries, the battery can be stored in this way for several years, but during this period the cell’s capacity will inevitably decrease.